Acta Biomaterialia ( IF 9.4 ) Pub Date : 2018-02-17 , DOI: 10.1016/j.actbio.2018.02.015 Xiao-Tao He 1 , Rui-Xin Wu 1 , Xin-Yue Xu 1 , Jia Wang 1 , Yuan Yin 1 , Fa-Ming Chen 1
|
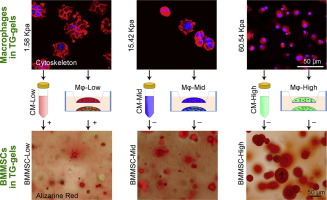
Accumulating evidence indicates that the physicochemical properties of biomaterials exert profound influences on stem cell fate decisions. However, matrix-based regulation selected through in vitro analyses based on a given cell population do not genuinely reflect the in vivo conditions, in which multiple cell types are involved and interact dynamically. This study constitutes the first investigation of how macrophages (Mφs) in stiffness-tunable transglutaminase cross-linked gelatin (TG-gel) affect the osteogenesis of bone marrow-derived mesenchymal stem cells (BMMSCs). When a single cell type was cultured, low-stiffness TG-gels promoted BMMSC proliferation, whereas high-stiffness TG-gels supported cell osteogenic differentiation. However, Mφs in high-stiffness TG-gels were more likely to polarize toward the pro-inflammatory M1 phenotype. Using either conditioned medium (CM)-based incubation or Transwell-based co-culture, we found that Mφs encapsulated in the low-stiffness matrix exerted a positive effect on the osteogenesis of co-cultured BMMSCs. Conversely, Mφs in high-stiffness TG-gels negatively affected cell osteogenic differentiation. When both cell types were cultured in the same TG-gel type and placed into the Transwell system, the stiffness-related influences of Mφs on BMMSCs were significantly altered; both the low- and high-stiffness matrices induced similar levels of BMMSC osteogenesis. Although the best material parameter for synergistically affecting Mφs and BMMSCs remains unknown, our data suggest that Mφ involvement in the co-culture system alters previously identified material-related influences on BMMSCs, such as matrix stiffness-related effects, which were identified based on a culture system involving a single cell type. Such Mφ-stem cell interactions should be considered when establishing proper matrix parameter-associated cell regulation in the development of biomimetic biomaterials for regenerative applications.
Statement of significance
The substrate stiffness of a scaffold plays critical roles in modulating both reparative cells, such as mesenchymal stem cells (MSCs), and immune cells, such as macrophages (Mφs). Although the influences of material stiffness on either Mφs or MSCs, have been extensively described, how the two cell types respond to matrix cues to dynamically affect each other in a three-dimensional (3D) biosystem remains largely unknown. Here, we report our findings that, in a platform wherein Mφs and bone marrow-derived MSCs coexist, matrix stiffness can influence stem cell fate through both direct matrix-associated regulation and indirect Mφ-based modulation. Our data support future studies of the MSC-Mφ-matrix interplay in the 3D context to optimize matrix parameters for the development of the next biomaterial.
中文翻译:

巨噬细胞参与影响三维培养条件下基质刚度对细胞成骨的影响
越来越多的证据表明,生物材料的物理化学性质对干细胞的命运决定产生深远的影响。然而,通过基于给定细胞群的体外分析选择的基于基质的调节并不能真正反映体内条件,其中涉及多种细胞类型并动态相互作用。本研究首次研究了刚度可调转谷氨酰胺酶交联明胶 (TG-gel) 中的巨噬细胞 (Mφs) 如何影响骨髓间充质干细胞 (BMMSCs) 的成骨。当培养单细胞类型时,低硬度 TG 凝胶促进 BMMSC 增殖,而高硬度 TG 凝胶支持细胞成骨分化。然而,高硬度 TG 凝胶中的 Mφs 更可能向促炎 M1 表型极化。使用基于条件培养基 (CM) 的孵育或基于 Transwell 的共培养,我们发现封装在低刚度基质中的 Mφs 对共培养的 BMMSCs 的成骨产生积极影响。反过来,高硬度 TG 凝胶中的 Mφs 对细胞成骨分化产生负面影响。当两种细胞类型在相同的 TG 凝胶类型中培养并放入 Transwell 系统时,Mφs 对 BMMSCs 的刚度相关影响显着改变;低刚度和高刚度基质均诱导相似水平的 BMMSC 成骨。尽管协同影响 Mφs 和 BMMSCs 的最佳材料参数仍然未知,但我们的数据表明,Mφ 参与共培养系统改变了先前确定的对 BMMSCs 的材料相关影响,例如基质刚度相关效应,这是基于涉及单一细胞类型的培养系统。
重要性声明
支架的底物刚度在调节修复细胞(如间充质干细胞 (MSCs))和免疫细胞(如巨噬细胞 (Mφs))中起关键作用。尽管材料刚度对 Mφs 或 MSCs 的影响已被广泛描述,但在三维 (3D) 生物系统中,这两种细胞类型如何响应基质线索以动态地相互影响仍然很大程度上未知。在这里,我们报告了我们的发现,在 Mφs 和骨髓来源的 MSCs 共存的平台中,基质刚度可以通过直接的基质相关调节和间接的基于 Mφ 的调节来影响干细胞的命运。我们的数据支持未来在 3D 环境中研究 MSC-Mφ 基质相互作用,以优化基质参数以开发下一种生物材料。











































 京公网安备 11010802027423号
京公网安备 11010802027423号