Electrochemistry Communications ( IF 4.7 ) Pub Date : 2018-02-17 , DOI: 10.1016/j.elecom.2018.02.009 Xia Qing Zuo , Wei Chen , Anni Yu , Mian Le Xu , Jun Cai , Yan-Xia Chen
|
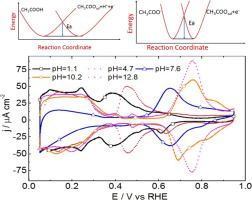
pH effect on thermodynamics and kinetics of acetate adsorption on the Pt(111) electrode in solutions is studied over a wide pH range (from 1 to 13) by cyclic voltammetry and electrochemical impedance spectroscopy. With increasing solution pH i) from pH = 1.1 to 4.7, the peak potential for acetate adsorption shifts slightly from 0.39 VRHE to 0.46 VRHE, ii) from pH = 4.7 to 10.2 it shifts by ca. 56 mV/pH unit; iii) the adsorption isotherm for acetate adsorption does not show obvious changes, but the adsorption kinetics become slower with pH changing from 1.1, 4.7 to 7.6. The positive shift in the acetate adsorption peak with pH (on the RHE scale) can be explained by the pH-dependence of the thermodynamic equilibrium potential for acetate adsorption with either acetic acid or acetate anions as the precursor. The faster acetate adsorption kinetics in acid than in alkaline solution are attributed to a reduced activation barrier, owing to the formation of hydrated H+ upon CH3COOH deprotonation to adsorbed acetate.
中文翻译:

pH对Pt(111)电极上乙酸盐吸附的影响
通过循环伏安法和电化学阻抗谱研究了pH对乙酸盐在溶液中Pt(111)电极上吸附乙酸的热力学和动力学的影响,该溶液在较宽的pH范围内(从1到13)进行了研究。随着溶液pH i)从pH = 1.1增加到4.7,乙酸盐吸附的峰值电势从0.39 V RHE轻微移至0.46 V RHE,ii)在pH = 4.7至10.2的范围内移动了大约。56 mV / pH单位; iii)乙酸盐吸附的吸附等温线没有显示出明显的变化,但是随着pH从1.1、4.7到7.6的变化,吸附动力学变慢了。乙酸盐吸附峰随pH值(在RHE尺度上)的正移可以用乙酸或乙酸根阴离子作为前体的乙酸盐吸附的热力学平衡势的pH依赖性来解释。在酸中,与在碱性溶液中相比,乙酸在乙酸中的吸附动力学更快,这归因于活化障碍的减少,这是由于在CH 3 COOH脱质子化为吸附的乙酸时形成了水合H +。











































 京公网安备 11010802027423号
京公网安备 11010802027423号