当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
From a Highly Disordered to a Metastable State: Uncovering Insights of α-Synuclein
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2018-02-16 00:00:00 , DOI: 10.1021/acschemneuro.7b00446 Yoann Cote 1, 2 , Patrice Delarue 2 , Harold A. Scheraga 3 , Patrick Senet 2, 3 , Gia G. Maisuradze 3
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2018-02-16 00:00:00 , DOI: 10.1021/acschemneuro.7b00446 Yoann Cote 1, 2 , Patrice Delarue 2 , Harold A. Scheraga 3 , Patrick Senet 2, 3 , Gia G. Maisuradze 3
Affiliation
|
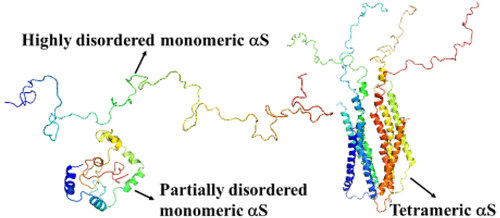
α-Synuclein (αS) is a major constituent of Lewy bodies, the insoluble aggregates that are the hallmark of one of the most prevalent neurodegenerative disorders, Parkinson’s disease (PD). The vast majority of experiments in vitro and in vivo provide extensive evidence that a disordered monomeric form is the predominant state of αS in water solution, and it undergoes a large-scale disorder-to-helix transition upon binding to vesicles of different types. Recently, another form, tetrameric, of αS with a stable helical structure was identified experimentally. It has been shown that a dynamic intracellular population of metastable αS tetramers and monomers coexists normally; and the tetramer plays an essential role in maintaining αS homeostasis. Therefore, it is of interest to know whether the tetramer can serve as a means of preventing or delaying the start of PD. Before answering this very important question, it is, first, necessary to find out, on an atomistic level, a correlation between tetramers and monomers; what mediates tetramer formation and what makes a tetramer stable. We address these questions here by investigating both monomeric and tetrameric forms of αS. In particular, by examining correlations between the motions of the side chains and the main chain, steric parameters along the amino-acid sequence, and one- and two-dimensional free-energy landscapes along the coarse-grained dihedral angles γ and δ and principal components, respectively, in monomeric and tetrameric αS, we were able to shed light on a fundamental relationship between monomers and tetramers, and the key residues involved in mediating formation of a tetramer. Also, the reasons for the stability of tetrameric αS and inability of monomeric αS to fold are elucidated here.
中文翻译:

从高度混乱到亚稳态:发现α-突触核蛋白的见解
α-突触核蛋白(αS)是路易体的主要成分,不溶性聚集体是最普遍的神经退行性疾病之一帕金森氏病(PD)的标志。体外和体内的绝大多数实验提供了广泛的证据,表明无序单体形式是水溶液中αS的主要状态,并且与不同类型的囊泡结合后会经历大规模的无序-螺旋转变。最近,通过实验确定了另一种形式的具有稳定螺旋结构的αS四聚体。已经表明,动态稳定的αS四聚体和单体的动态细胞内群体正常共存。四聚体在维持αS稳态中起着至关重要的作用。所以,有趣的是要知道四聚体是否可以用作预防或延迟PD启动的手段。在回答这个非常重要的问题之前,首先,必须在原子水平上找出四聚体与单体之间的相关性。什么介导四聚体形成以及什么使四聚体稳定。我们在这里通过研究αS的单体和四聚体形式来解决这些问题。特别是,通过检查侧链和主链运动之间的相关性,氨基酸序列的空间参数以及沿粗粒二面角γ和δ以及主体的一维和二维自由能态势分别在单体和四聚体αS中的组分,我们得以阐明单体与四聚体之间的基本关系,以及涉及介导四聚体形成的关键残基。另外,在此阐明了四聚体αS的稳定性和单体αS不能折叠的原因。
更新日期:2018-02-16
中文翻译:

从高度混乱到亚稳态:发现α-突触核蛋白的见解
α-突触核蛋白(αS)是路易体的主要成分,不溶性聚集体是最普遍的神经退行性疾病之一帕金森氏病(PD)的标志。体外和体内的绝大多数实验提供了广泛的证据,表明无序单体形式是水溶液中αS的主要状态,并且与不同类型的囊泡结合后会经历大规模的无序-螺旋转变。最近,通过实验确定了另一种形式的具有稳定螺旋结构的αS四聚体。已经表明,动态稳定的αS四聚体和单体的动态细胞内群体正常共存。四聚体在维持αS稳态中起着至关重要的作用。所以,有趣的是要知道四聚体是否可以用作预防或延迟PD启动的手段。在回答这个非常重要的问题之前,首先,必须在原子水平上找出四聚体与单体之间的相关性。什么介导四聚体形成以及什么使四聚体稳定。我们在这里通过研究αS的单体和四聚体形式来解决这些问题。特别是,通过检查侧链和主链运动之间的相关性,氨基酸序列的空间参数以及沿粗粒二面角γ和δ以及主体的一维和二维自由能态势分别在单体和四聚体αS中的组分,我们得以阐明单体与四聚体之间的基本关系,以及涉及介导四聚体形成的关键残基。另外,在此阐明了四聚体αS的稳定性和单体αS不能折叠的原因。



























 京公网安备 11010802027423号
京公网安备 11010802027423号