Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-02-16 , DOI: 10.1016/j.bioorg.2018.02.016 Jiayue Xi , Siyuan Xu , Lulu Zhang , Xueyuan Bi , Yanshen Ren , Yu-Chih Liu , Yueqing Gu , Yungen Xu , Fei Lan , Xiaoming Zha
|
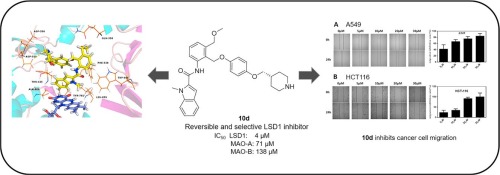
Lysine specific demethylase 1 (LSD1) plays a vital role in epigenetic regulation of gene activation and repression in several human cancers and is recognized as a promising antitumor therapeutic target. In this paper, a series of 4-(4-benzyloxy)phenoxypiperidines were synthesized and evaluated. Among the tested compounds, compound 10d exhibited the potent and reversible inhibitory activity against LSD1 in vitro (IC50 = 4 μM). Molecular docking was conducted to predict its binding mode. Furthermore, 10d displayed it could inhibit migration of HCT-116 colon cancer cells and A549 lung cancer cells. Taken together, 10d deserves further investigation as a hit-to-lead for the treatment of LSD1 associated tumors.
中文翻译:

设计,合成和生物活性的4-(4-苄氧基)苯氧基哌啶作为选择性和可逆的LSD1抑制剂
赖氨酸特异性脱甲基酶1(LSD1)在几种人类癌症的表观遗传调控基因激活和抑制中起着至关重要的作用,被认为是一种有前途的抗肿瘤治疗靶标。本文合成并评估了一系列的4-(4-苄氧基)苯氧基哌啶。在测试的化合物中,化合物10d在体外表现出对LSD1的有效且可逆的抑制活性(IC 50 = 4μM)。进行分子对接以预测其结合模式。此外,第10天显示它可以抑制HCT-116结肠癌细胞和A549肺癌细胞的迁移。两者合计,10d作为治疗LSD1相关肿瘤的首选药物值得进一步研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号