Molecular Catalysis ( IF 3.9 ) Pub Date : 2018-02-16 , DOI: 10.1016/j.mcat.2018.02.006 Liangliang Zhang , Xiao Chen , Zhijian Peng , Changhai Liang
|
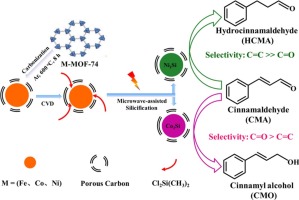
Chemoselective hydrogenation of CC and C
O bonds in the cinnamaldehyde (CMA), was successfully performed with highly dispersed transition metal silicides-embedded in the porous carbon matrix (M2[email protected] (M = Fe, Co, Ni)) nanocatalysts with a particle size of 8–12 nm, which were synthesized by a facile approach via microwave-assisted chemical vapor deposition (MWCVD) using organosilane and metal-organic-framework (MOFs-74)-templated porous carbon matrix-encapsulated transition metal ([email protected]) precursors. Compared with [email protected], the activity of M2[email protected] (M = Fe, Co, Ni) nanocatalysts obviously increases, which can be attributed that the overlayered carbon on metals has been destroyed during MWCVD processing, leading to the exposure of more active sites of catalysts. Due to the inherent electronic properties of metallic active sites in the metal silicides, the catalytic activity of Ni2[email protected] was much higher than that of Co2[email protected] and Fe2[email protected] in the chemoselective hydrogenation of CMA. At total conversion of CMA, the Co2[email protected] nanocatalyst was chemoselective for the hydrogenation of polar C
O bonds (selectivity to cinnamyl alcohol of ∼60%), whereas Ni2[email protected] nanocatalyst was highly chemoselective for the hydrogenation of non-polar C
C bonds (selectivity to hydrocinnamaldehyde of ∼90%). The doping of silicon atoms with more electropositive into metals lattices in the M-Si intermetallic compounds (IMCs) influenced the adsorption of substrate molecule on the catalyst surface, slightly leading to the different reaction routes. In addition, the Co2[email protected] and Ni2[email protected] respectively maintain the robust stability in the hydrogenation of CMA. This result provided guidance on the design of catalyst via tuning the polarization properties of the inter-atoms in the IMCs according to the target product.
中文翻译:

在MOF衍生的M 2 [受电子邮件保护](M = Fe,Co,Ni)硅化物催化剂上对肉桂醛进行化学选择性加氢
使用高度分散的过渡金属硅化物嵌入多孔碳基质(M 2 [受电子邮件保护](M = Fe,Co,Ni))纳米催化剂中,成功地进行了肉桂醛(CMA)中C C和C
O键的化学选择性氢化。粒径为8–12 nm,通过使用有机硅烷和金属-有机骨架(MOFs-74)-模板多孔碳基质封装的过渡金属通过微波辅助化学气相沉积(MWCVD)的简便方法合成( [受电子邮件保护])前体。与[电子邮件保护]相比,M 2的活动[电子邮件保护](M = Fe,Co,Ni)纳米催化剂明显增加,这可以归因于金属的上覆碳在MWCVD处理过程中已被破坏,从而导致了更多活性位点的暴露。由于金属硅化物中金属活性位的固有电子性质,在CMA的化学选择性加氢中,Ni 2 [受电子邮件保护]和Co 2 [受电子邮件保护]和Fe 2 [受电子邮件保护]的催化活性要高得多。 。在CMA的总转化率下,Co 2 [受电子邮件保护的]纳米催化剂对极性C
O键的氢化具有化学选择性(对肉桂醇的选择性约为60%),而Ni 2[电子邮件保护的]纳米催化剂对非极性C
C键的氢化具有高度的化学选择性(对氢肉桂醛的选择性约为90%)。在M-Si金属间化合物(IMC)中,具有更正电性的硅原子掺杂到金属晶格中会影响底物分子在催化剂表面的吸附,从而略有不同的反应路线。此外,Co 2 [受电子邮件保护]和Ni 2 [受电子邮件保护]分别在CMA加氢中保持了稳固的稳定性。该结果通过根据目标产物调节IMC中原子间原子的极化性质,为催化剂的设计提供了指导。










































 京公网安备 11010802027423号
京公网安备 11010802027423号