当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spectroelectrochemical studies of a ruthenium complex containing the pH sensitive 4,4′-dihydroxy-2,2′-bipyridine ligand†
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-02-16 00:00:00 , DOI: 10.1039/c7dt04554a Erin J. Viere 1, 2, 3, 4 , Ashley E. Kuhn 1, 2, 3, 4 , Margaret H. Roeder 1, 2, 3, 4 , Nicholas A. Piro 1, 5, 6, 7 , W. Scott Kassel 1, 2, 3, 4 , Timothy J. Dudley 7, 8, 9, 10, 11 , Jared J. Paul 1, 2, 3, 4
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-02-16 00:00:00 , DOI: 10.1039/c7dt04554a Erin J. Viere 1, 2, 3, 4 , Ashley E. Kuhn 1, 2, 3, 4 , Margaret H. Roeder 1, 2, 3, 4 , Nicholas A. Piro 1, 5, 6, 7 , W. Scott Kassel 1, 2, 3, 4 , Timothy J. Dudley 7, 8, 9, 10, 11 , Jared J. Paul 1, 2, 3, 4
Affiliation
|
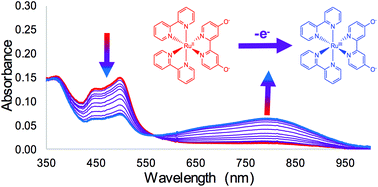
Attaining high oxidation states at the metal center of transition metal complexes is a key design principle for many catalytic processes. One way to support high oxidation state chemistry is to utilize ligands that are electron-donating in nature. Understanding the structural and electronic changes of metal complexes as higher oxidation states are reached is critical towards designing more robust catalysts that are able to turn over at high rates without decomposing. To this end, we report herein the changes in structural and electronic properties as [Ru(bpy)2(44′bpy(OH)2)]2+ is oxidized to [Ru(bpy)2(44′bpy(OH)2)]3+ (bpy = 2,2′-bipyridine; 44′bpy(OH)2 = 4,4′-dihydroxy-2,2′-bipyridine). The 44′bpy(OH)2 ligand is a pH-dependent ligand where deprotonation of the hydroxyl groups leads to significant electronic donation to the metal center. A Pourbaix Diagram of the complex reveals a pH independent reduction potential below pH = 2.0 for the Ru3+/2+ process at 0.91 V vs. Ag/AgCl. Above pH = 2.0, pH dependence is observed with a decrease in reduction potential until pH = 6.8 where the complex is completely deprotonated, resulting in a reduction potential of 0.62 V vs. Ag/AgCl. Spectroelectrochemical studies as a function of pH reveal the disappearance of the Metal to Ligand Charge Transfer (MLCT) or Mixed Metal–Ligand to Charge Transfer bands upon oxidation and the appearance of a new low energy band. DFT calculations for this low energy band were carried out using both B3LYP and M06-L functionals for all protonation states and suggest that numerous new transition types occur upon oxidation to Ru3+.
中文翻译:

含pH敏感的4,4'-二羟基-2,2'-联吡啶配体的钌配合物的光谱电化学研究†
在许多催化过程中,在过渡金属络合物的金属中心处获得高氧化态是关键的设计原理。支持高氧化态化学的一种方法是利用本质上是给电子体的配体。了解金属配合物在达到更高氧化态时的结构和电子变化,对于设计出能够在不分解的情况下以高速率翻转的更坚固的催化剂至关重要。为此,我们报告本文中的结构和电子性质的变化的[Ru(联吡啶)2(44'bpy(OH)2)] 2+被氧化成的[Ru(联吡啶)2(44'bpy(OH)2)] 3+(bpy = 2,2'-联吡啶; 44'bpy(OH)2= 4,4'-二羟基-2,2'-联吡啶)。44'bpy(OH)2配体是pH依赖的配体,其中羟基的去质子化导致向金属中心的大量电子供体。该络合物的Pourbaix图显示了在相对于Ag / AgCl为0.91 V时Ru 3 + / 2 +过程的pH = 2.0以下,与pH无关的还原电位。高于pH = 2.0时,观察到pH依赖性,还原电位降低,直到pH = 6.8处复合物完全去质子化,导致还原电位为0.62 V vs.。Ag / AgCl。光谱电化学研究随pH的变化揭示了金属到配体电荷转移带(MLCT)或混合的金属-配体到电荷转移带在氧化时消失,并出现了新的低能带。使用所有质子化态的B3LYP和M06-L官能团对这一低能带进行DFT计算,结果表明在氧化为Ru 3+时会出现许多新的过渡类型。
更新日期:2018-02-16
中文翻译:

含pH敏感的4,4'-二羟基-2,2'-联吡啶配体的钌配合物的光谱电化学研究†
在许多催化过程中,在过渡金属络合物的金属中心处获得高氧化态是关键的设计原理。支持高氧化态化学的一种方法是利用本质上是给电子体的配体。了解金属配合物在达到更高氧化态时的结构和电子变化,对于设计出能够在不分解的情况下以高速率翻转的更坚固的催化剂至关重要。为此,我们报告本文中的结构和电子性质的变化的[Ru(联吡啶)2(44'bpy(OH)2)] 2+被氧化成的[Ru(联吡啶)2(44'bpy(OH)2)] 3+(bpy = 2,2'-联吡啶; 44'bpy(OH)2= 4,4'-二羟基-2,2'-联吡啶)。44'bpy(OH)2配体是pH依赖的配体,其中羟基的去质子化导致向金属中心的大量电子供体。该络合物的Pourbaix图显示了在相对于Ag / AgCl为0.91 V时Ru 3 + / 2 +过程的pH = 2.0以下,与pH无关的还原电位。高于pH = 2.0时,观察到pH依赖性,还原电位降低,直到pH = 6.8处复合物完全去质子化,导致还原电位为0.62 V vs.。Ag / AgCl。光谱电化学研究随pH的变化揭示了金属到配体电荷转移带(MLCT)或混合的金属-配体到电荷转移带在氧化时消失,并出现了新的低能带。使用所有质子化态的B3LYP和M06-L官能团对这一低能带进行DFT计算,结果表明在氧化为Ru 3+时会出现许多新的过渡类型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号