当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sigmatropic Rearrangements of Hypervalent‐Iodine‐Tethered Intermediates for the Synthesis of Biaryls
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-03-13 , DOI: 10.1002/anie.201801132 Mitsuki Hori 1 , Jing-Dong Guo 2 , Tomoyuki Yanagi 1 , Keisuke Nogi 1 , Takahiro Sasamori 3 , Hideki Yorimitsu 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-03-13 , DOI: 10.1002/anie.201801132 Mitsuki Hori 1 , Jing-Dong Guo 2 , Tomoyuki Yanagi 1 , Keisuke Nogi 1 , Takahiro Sasamori 3 , Hideki Yorimitsu 1
Affiliation
|
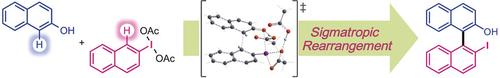
Metal‐free dehydrogenative couplings of aryliodanes with phenols to afford 2‐hydroxy‐2′‐iodobiaryls have been developed. This reaction proceeds through ligand exchange on the hypervalent iodine atom followed by a [3,3] sigmatropic rearrangement and with complete regioselectivity. This coupling, in combination with in situ oxidation by mCPBA, enables the convenient conversion of iodoarenes into desirable biaryls. The obtained biaryls have convertible iodo and hydroxy groups in close proximity, and are thus synthetically useful, as exemplified by the controlled syntheses of π‐extended furans and a substituted [5]helicene. DFT calculations clearly revealed that the rearrangement is sigmatropic, with C−C bond formation and I−O bond cleavage proceeding in a concerted manner. Acetic acid, which was found to be the best solvent for this protocol, renders the iodine atom more cationic and thus accelerates the sigmatropic rearrangement.
中文翻译:

高价碘拴系中间体的适马重排,用于合成联芳基
已开发了芳金属芳烃与苯酚的无金属脱氢偶联反应,可制得2-羟基-2'-碘联芳基。该反应通过高价碘原子上的配体交换进行,然后进行[3,3]σ重排,并具有完全的区域选择性。这种耦合,结合在原位氧化由米CPBA使碘代芳烃方便地转化为所需的联芳基。所获得的联芳基在附近具有可转换的碘基和羟基,因此可用于合成,如π延伸的呋喃和取代的[5]螺旋烯的受控合成所举例说明的。DFT计算清楚地表明,重排是σ向性的,CC键的形成和IO键的断裂均以协调的方式进行。乙酸是该方案的最佳溶剂,它使碘原子更具阳离子性,从而加速了σ重排。
更新日期:2018-03-13
中文翻译:

高价碘拴系中间体的适马重排,用于合成联芳基
已开发了芳金属芳烃与苯酚的无金属脱氢偶联反应,可制得2-羟基-2'-碘联芳基。该反应通过高价碘原子上的配体交换进行,然后进行[3,3]σ重排,并具有完全的区域选择性。这种耦合,结合在原位氧化由米CPBA使碘代芳烃方便地转化为所需的联芳基。所获得的联芳基在附近具有可转换的碘基和羟基,因此可用于合成,如π延伸的呋喃和取代的[5]螺旋烯的受控合成所举例说明的。DFT计算清楚地表明,重排是σ向性的,CC键的形成和IO键的断裂均以协调的方式进行。乙酸是该方案的最佳溶剂,它使碘原子更具阳离子性,从而加速了σ重排。











































 京公网安备 11010802027423号
京公网安备 11010802027423号