当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evidence that Crystal Facet Orientation Dictates Oxygen Evolution Intermediates on Rutile Manganese Oxide
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2018-02-16 , DOI: 10.1002/adfm.201706319 Hirotaka Kakizaki 1, 2 , Hideshi Ooka 1, 2 , Toru Hayashi 1, 2 , Akira Yamaguchi 1 , Nadège Bonnet‐Mercier 1 , Kazuhito Hashimoto 3 , Ryuhei Nakamura 1, 4
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2018-02-16 , DOI: 10.1002/adfm.201706319 Hirotaka Kakizaki 1, 2 , Hideshi Ooka 1, 2 , Toru Hayashi 1, 2 , Akira Yamaguchi 1 , Nadège Bonnet‐Mercier 1 , Kazuhito Hashimoto 3 , Ryuhei Nakamura 1, 4
Affiliation
|
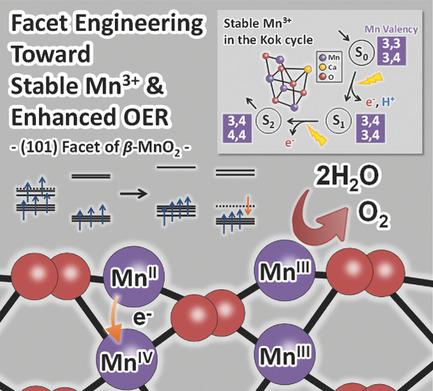
Elucidating the mechanism that differentiates the oxygen‐evolving center of photosystem II with its inorganic counterpart is crucial to develop efficient catalysts for the oxygen evolution reaction (OER). Previous studies have suggested that the larger overpotential for MnO2 catalysts under neutral conditions may result from the instability of the Mn3+ intermediate to charge disproportionation. Here, by monitoring the surface intermediates of electrochemical OER on rutile MnO2 with different facet orientations, a correlation between the stability of the intermediate species and crystal facets is confirmed explicitly for the first time. The coverage of the Mn3+ intermediate is found to be 11‐fold higher on the metastable (101) surfaces compared to (110) surfaces, leading to the superior OER activity of (101) surfaces. The difference in OER activity may result from the difference in surface electronic states of Mn3+, where interlayer charge comproportionation of Mn2+ and Mn4+ to generate two Mn3+ species is favored on (101) facets. Considering the fact that the OER enzyme accommodates Mn3+ stably during the Kok cycle, the enhanced OER activity of the rutile MnO2 catalyst with a metastable surface highlights the importance of mimicking not only the crystal structure but also the electronic structure of the targeted natural enzyme.
中文翻译:

晶体刻面取向指示氧气在金红石型氧化锰上的介导作用的证据
阐明区分光系统II的放氧中心与其无机对应物的机理对于开发用于放氧反应(OER)的高效催化剂至关重要。先前的研究表明,MnO 2催化剂在中性条件下的更大的超电势可能是由于Mn 3+中间体电荷歧化的不稳定性所致。在此,通过监测具有不同刻面取向的金红石型MnO 2上的电化学OER的表面中间产物,首次明确证实了中间物质的稳定性与晶体刻面之间的相关性。Mn 3+的覆盖率与(110)表面相比,亚稳态(101)表面的中间体的含量高11倍,从而导致(101)表面具有优异的OER活性。OER活性的差异可能是由于Mn 3+的表面电子状态不同所致,其中在(101)面上有利于Mn 2+和Mn 4+的层间电荷互补以生成两个Mn 3+物种。考虑到OER酶在Kok循环中稳定地容纳Mn 3+的事实,具有亚稳表面的金红石MnO 2催化剂增强的OER活性突出了不仅模拟目标天然结构的晶体结构而且电子结构的重要性。酶。
更新日期:2018-02-16
中文翻译:

晶体刻面取向指示氧气在金红石型氧化锰上的介导作用的证据
阐明区分光系统II的放氧中心与其无机对应物的机理对于开发用于放氧反应(OER)的高效催化剂至关重要。先前的研究表明,MnO 2催化剂在中性条件下的更大的超电势可能是由于Mn 3+中间体电荷歧化的不稳定性所致。在此,通过监测具有不同刻面取向的金红石型MnO 2上的电化学OER的表面中间产物,首次明确证实了中间物质的稳定性与晶体刻面之间的相关性。Mn 3+的覆盖率与(110)表面相比,亚稳态(101)表面的中间体的含量高11倍,从而导致(101)表面具有优异的OER活性。OER活性的差异可能是由于Mn 3+的表面电子状态不同所致,其中在(101)面上有利于Mn 2+和Mn 4+的层间电荷互补以生成两个Mn 3+物种。考虑到OER酶在Kok循环中稳定地容纳Mn 3+的事实,具有亚稳表面的金红石MnO 2催化剂增强的OER活性突出了不仅模拟目标天然结构的晶体结构而且电子结构的重要性。酶。











































 京公网安备 11010802027423号
京公网安备 11010802027423号