当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Aspects of Pincer Nickel(II)-Catalyzed C–H Bond Alkylation of Azoles with Alkyl Halides
Organometallics ( IF 2.5 ) Pub Date : 2018-02-15 00:00:00 , DOI: 10.1021/acs.organomet.8b00025 Ulhas N. Patel 1 , Shailja Jain , Dilip K. Pandey 1 , Rajesh G. Gonnade , Kumar Vanka , Benudhar Punji 1
Organometallics ( IF 2.5 ) Pub Date : 2018-02-15 00:00:00 , DOI: 10.1021/acs.organomet.8b00025 Ulhas N. Patel 1 , Shailja Jain , Dilip K. Pandey 1 , Rajesh G. Gonnade , Kumar Vanka , Benudhar Punji 1
Affiliation
|
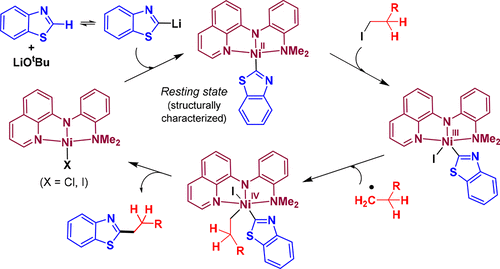
The quinolinyl-based pincer nickel complex, κN,κN,κN-{C9H6N-(μ-N)-C6H4–NMe2}NiCl [(QNNNMe2)NiCl; (1)] has recently been demonstrated to be an efficient and robust catalyst for the alkylation of azoles with alkyl halides under copper-free conditions. Herein, we report the detailed mechanistic investigation for the alkylation of azoles catalyzed by (QNNNMe2)NiCl (1), which highlights an iodine-atom transfer (IAT) mechanism for the reaction involving a NiII/NiIII process. Deuterium labeling experiments indicate reversible cleavage of the benzothiazole C–H bond, and kinetic studies underline a fractional negative rate order with the substrate benzothiazole. The involvement of an alkyl radical during the alkylation is validated by radical clock and external additive experiments. An active intermediate species (QNNNMe2)Ni(benzothiazolyl) (5a) has been isolated and structurally characterized. The complex (QNNNMe2)Ni(benzothiazolyl) (5a) is found to be the resting state of catalyst 1. Kinetic analysis of electronically different intermediates suggests that the step involving the reaction of 5a with alkyl iodide is crucial and a rate-influencing step. DFT calculations strongly support the experimental findings and corroborate an IAT process for the alkylation reaction.
中文翻译:

钳制镍(II)催化偶氮与烷基卤化物的C–H键烷基化的机理
基于喹啉基钳形镍络合物,κ Ñ,κ Ñ,κ ñ - {C 9 ħ 6 N-(μ-N)-C 6 H ^ 4 -NMe 2 }的NiCl [(Q NNN ME2)的NiCl; (1)]最近被证明是一种在无铜条件下用烷基卤化物将唑类烷基化的有效而稳定的催化剂。在此,我们报告了由(Q NNN Me2)NiCl(1)催化的唑烷基化的详细机理研究,该研究着重介绍了涉及Ni II的反应的碘原子转移(IAT)机理。/ Ni III工艺。氘标记实验表明苯并噻唑CH键可逆裂解,动力学研究强调了底物苯并噻唑的分数负速率顺序。通过自由基钟和外部添加剂实验验证了烷基在烷基化过程中的参与。活性中间物种(Q NNN Me2)Ni(苯并噻唑基)(5a)已被分离出来,并在结构上进行了表征。发现络合物(Q NNN Me2)Ni(苯并噻唑基)(5a)是催化剂1的静止状态。电子不同中间体的动力学分析表明,涉及5a与烷基碘反应的步骤至关重要,并且是影响速率的步骤。DFT计算有力地支持了实验结果,并证实了用于烷基化反应的IAT工艺。
更新日期:2018-02-16
中文翻译:

钳制镍(II)催化偶氮与烷基卤化物的C–H键烷基化的机理
基于喹啉基钳形镍络合物,κ Ñ,κ Ñ,κ ñ - {C 9 ħ 6 N-(μ-N)-C 6 H ^ 4 -NMe 2 }的NiCl [(Q NNN ME2)的NiCl; (1)]最近被证明是一种在无铜条件下用烷基卤化物将唑类烷基化的有效而稳定的催化剂。在此,我们报告了由(Q NNN Me2)NiCl(1)催化的唑烷基化的详细机理研究,该研究着重介绍了涉及Ni II的反应的碘原子转移(IAT)机理。/ Ni III工艺。氘标记实验表明苯并噻唑CH键可逆裂解,动力学研究强调了底物苯并噻唑的分数负速率顺序。通过自由基钟和外部添加剂实验验证了烷基在烷基化过程中的参与。活性中间物种(Q NNN Me2)Ni(苯并噻唑基)(5a)已被分离出来,并在结构上进行了表征。发现络合物(Q NNN Me2)Ni(苯并噻唑基)(5a)是催化剂1的静止状态。电子不同中间体的动力学分析表明,涉及5a与烷基碘反应的步骤至关重要,并且是影响速率的步骤。DFT计算有力地支持了实验结果,并证实了用于烷基化反应的IAT工艺。










































 京公网安备 11010802027423号
京公网安备 11010802027423号