当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lewis acid‐mediated defluorinative [3+2] cycloaddition/aromatization cascade of 2,2‐difluoroethanol systems with nitriles
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-02-28 , DOI: 10.1002/adsc.201701581 Min-Tsang Hsieh,Kuo-Hsiung Lee,Sheng-Chu Kuo,Hui-Chang Lin
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-02-28 , DOI: 10.1002/adsc.201701581 Min-Tsang Hsieh,Kuo-Hsiung Lee,Sheng-Chu Kuo,Hui-Chang Lin
|
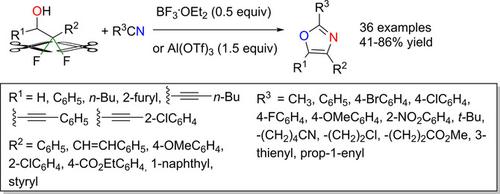
The properties of C−F bonds, including high thermal and chemical stability, make derivatization of organic fluorine‐containing compounds by the activation of the C−F bond and subsequent functionalization quite challenging. We herein report a Lewis acid‐mediated defluorinative cycloaddition/aromatization cascade of 2,2‐difluoroethanols with nitriles as a novel synthetic method for the preparation of 2,4,5‐trisubstituted oxazoles. This reaction, which involves cleavage of two C−F bonds and the consecutive formation of C−O and C−N bonds in a one‐pot fashion, features a broad substrate scope and moderate to high reaction yields. Mechanistic studies revealed that the reaction is initiated by the Lewis acid‐mediated ring closure of the 2,2‐difluoroethanol to produce the fluoroepoxide intermediate.
中文翻译:

Lewis酸介导的含腈的2,2-二氟乙醇体系的除氟[3 + 2]环加成/芳构化级联反应
C-F键的特性(包括高的热稳定性和化学稳定性)使C-F键的活化和随后的功能化变得非常困难,从而使有机含氟化合物衍生化。我们在本文中报道了一种2,9,5-二氟乙醇与腈的路易斯酸介导的脱氟环加成/芳构化级联反应,作为制备2,4,5-三取代的恶唑的一种新型合成方法。该反应涉及两个碳原子键的断裂以及单键连续形成碳原子键和碳原子键的反应,具有广泛的底物范围和中等至高的反应产率。机理研究表明,该反应是由路易斯酸介导的2,2-二氟乙醇的闭环反应产生的,是氟代环氧中间体。
更新日期:2018-02-28
中文翻译:

Lewis酸介导的含腈的2,2-二氟乙醇体系的除氟[3 + 2]环加成/芳构化级联反应
C-F键的特性(包括高的热稳定性和化学稳定性)使C-F键的活化和随后的功能化变得非常困难,从而使有机含氟化合物衍生化。我们在本文中报道了一种2,9,5-二氟乙醇与腈的路易斯酸介导的脱氟环加成/芳构化级联反应,作为制备2,4,5-三取代的恶唑的一种新型合成方法。该反应涉及两个碳原子键的断裂以及单键连续形成碳原子键和碳原子键的反应,具有广泛的底物范围和中等至高的反应产率。机理研究表明,该反应是由路易斯酸介导的2,2-二氟乙醇的闭环反应产生的,是氟代环氧中间体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号