当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Promoting Ethylene Selectivity from CO2 Electroreduction on CuO Supported onto CO2 Capture Materials
ChemSusChem ( IF 7.5 ) Pub Date : 2018-02-15 , DOI: 10.1002/cssc.201702338 Hui-Juan Yang 1 , Hong Yang 1 , Yu-Hao Hong 1 , Peng-Yang Zhang 1 , Tao Wang 1 , Li-Na Chen 1 , Feng-Yang Zhang 1 , Qi-Hui Wu 2 , Na Tian 1 , Zhi-You Zhou 1 , Shi-Gang Sun 1
ChemSusChem ( IF 7.5 ) Pub Date : 2018-02-15 , DOI: 10.1002/cssc.201702338 Hui-Juan Yang 1 , Hong Yang 1 , Yu-Hao Hong 1 , Peng-Yang Zhang 1 , Tao Wang 1 , Li-Na Chen 1 , Feng-Yang Zhang 1 , Qi-Hui Wu 2 , Na Tian 1 , Zhi-You Zhou 1 , Shi-Gang Sun 1
Affiliation
|
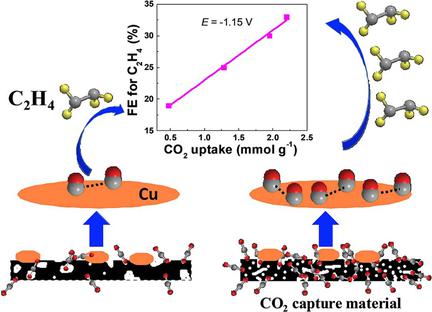
Cu is a unique catalyst for CO2 electroreduction, since it can catalyze CO2 reduction to a series of hydrocarbons, alcohols, and carboxylic acids. Nevertheless, such Cu catalysts suffer from poor selectivity. High pressure of CO2 is considered to facilitate the activity and selectivity of CO2 reduction. Herein, a new strategy is presented for CO2 reduction with improved C2H4 selectivity on a Cu catalyst by using CO2 capture materials as the support at ambient pressure. N‐doped carbon (NxC) was synthesized through high‐temperature carbonization of melamine and l‐lysine. We observed that the CO2 uptake capacity of NxC depends on both the microporous area and the content of pyridinic N species, which can be controlled by the carbonization temperature (600–800 °C). The as‐prepared CuO/NxC catalysts exhibit a considerably higher C2H4 faradaic efficiency (36 %) than CuO supported on XC‐72 carbon black (19 %), or unsupported CuO (20 %). Moreover, there is a good linear relationship between the C2H4 faradaic efficiency and CO2 uptake capacity of the supports for CuO. The local high CO2 concentration near Cu catalysts, created by CO2 capture materials, was proposed to increase the coverage of CO intermediate, which is favorable for the coupling of two CO units in the formation of C2H4. This study demonstrates that pairing Cu catalysts with CO2 capture supports is a promising approach for designing highly effective CO2 reduction electrocatalysts.
中文翻译:

负载在CO2捕集材料上的CuO上的CO2电解还原可提高乙烯的选择性
Cu是用于CO 2电解还原的独特催化剂,因为它可以催化将CO 2还原为一系列烃,醇和羧酸。然而,这种Cu催化剂的选择性差。认为CO 2的高压促进了CO 2还原的活性和选择性。在本文中,一种新的策略提出了用于CO 2还原改良过的C 2 ħ 4通过使用CO在Cu催化剂选择性2捕获材料在环境压力下的支持。N掺杂碳(N x C)是通过三聚氰胺和l的高温碳化而合成的赖氨酸。我们观察到,N x C的CO 2吸收能力取决于微孔面积和吡啶类N物种的含量,这可以通过碳化温度(600–800°C)来控制。所制备的CuO / N x C催化剂比XC-72炭黑负载的CuO(19%)或未负载的CuO(20%)表现出更高的C 2 H 4法拉第效率(36%)。此外,C 2 H 4法拉第效率和载体对CuO的CO 2吸收能力之间具有良好的线性关系。由CO 2产生的Cu催化剂附近的局部高CO 2浓度为了提高CO中间体的覆盖率,提出了一种捕集材料的方法,这有利于两个CO单元在C 2 H 4形成中的偶联。这项研究表明,将Cu催化剂与CO 2捕获载体配对是设计高效CO 2还原电催化剂的一种有前途的方法。
更新日期:2018-02-15
中文翻译:

负载在CO2捕集材料上的CuO上的CO2电解还原可提高乙烯的选择性
Cu是用于CO 2电解还原的独特催化剂,因为它可以催化将CO 2还原为一系列烃,醇和羧酸。然而,这种Cu催化剂的选择性差。认为CO 2的高压促进了CO 2还原的活性和选择性。在本文中,一种新的策略提出了用于CO 2还原改良过的C 2 ħ 4通过使用CO在Cu催化剂选择性2捕获材料在环境压力下的支持。N掺杂碳(N x C)是通过三聚氰胺和l的高温碳化而合成的赖氨酸。我们观察到,N x C的CO 2吸收能力取决于微孔面积和吡啶类N物种的含量,这可以通过碳化温度(600–800°C)来控制。所制备的CuO / N x C催化剂比XC-72炭黑负载的CuO(19%)或未负载的CuO(20%)表现出更高的C 2 H 4法拉第效率(36%)。此外,C 2 H 4法拉第效率和载体对CuO的CO 2吸收能力之间具有良好的线性关系。由CO 2产生的Cu催化剂附近的局部高CO 2浓度为了提高CO中间体的覆盖率,提出了一种捕集材料的方法,这有利于两个CO单元在C 2 H 4形成中的偶联。这项研究表明,将Cu催化剂与CO 2捕获载体配对是设计高效CO 2还原电催化剂的一种有前途的方法。









































 京公网安备 11010802027423号
京公网安备 11010802027423号