当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Peptoid Backbone Flexibilility Dictates Its Interaction with Water and Surfaces: A Molecular Dynamics Investigation
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1021/acs.biomac.7b01813 Arushi Prakash 1 , Marcel D. Baer 2 , Christopher J. Mundy 1, 2 , Jim Pfaendtner 1, 2
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1021/acs.biomac.7b01813 Arushi Prakash 1 , Marcel D. Baer 2 , Christopher J. Mundy 1, 2 , Jim Pfaendtner 1, 2
Affiliation
|
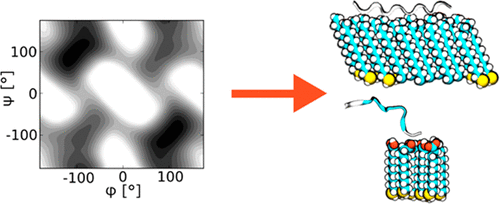
Peptoids are peptide-mimetic biopolymers that are easy to synthesize and adaptable for use in drugs, chemical scaffolds, and coatings. However, there is insufficient information about their structural preferences and interactions with the environment in various applications. We conducted a study to understand the fundamental differences between peptides and peptoids using molecular dynamics simulations with semiempirical (PM6) and empirical (AMBER) potentials, in conjunction with metadynamics enhanced sampling. From studies of single molecules in water and on surfaces, we found that sarcosine (model peptoid) is much more flexible than alanine (model peptide) in different environments. However, the sarcosine and alanine interact similarly with a hydrophobic or a hydrophilic. Finally, this study highlights the conformational landscape of peptoids and the dominant interactions that drive peptoids toward these conformations.
中文翻译:

拟肽骨架的柔韧性决定了它与水和表面的相互作用:分子动力学研究。
类肽是易于合成的肽模拟生物聚合物,适用于药物,化学支架和涂料。但是,在各种应用程序中,关于它们的结构偏好以及与环境的交互的信息不足。我们进行了一项研究,以利用具有半经验(PM6)和经验(AMBER)势能的分子动力学模拟,结合元动力学增强采样,来了解肽和类肽之间的基本差异。通过对水中和表面单分子的研究,我们发现在不同的环境中,肌氨酸(类肽模型)比丙氨酸(模型肽)具有更大的柔韧性。然而,肌氨酸和丙氨酸与疏水性或亲水性类似地相互作用。最后,
更新日期:2018-02-14
中文翻译:

拟肽骨架的柔韧性决定了它与水和表面的相互作用:分子动力学研究。
类肽是易于合成的肽模拟生物聚合物,适用于药物,化学支架和涂料。但是,在各种应用程序中,关于它们的结构偏好以及与环境的交互的信息不足。我们进行了一项研究,以利用具有半经验(PM6)和经验(AMBER)势能的分子动力学模拟,结合元动力学增强采样,来了解肽和类肽之间的基本差异。通过对水中和表面单分子的研究,我们发现在不同的环境中,肌氨酸(类肽模型)比丙氨酸(模型肽)具有更大的柔韧性。然而,肌氨酸和丙氨酸与疏水性或亲水性类似地相互作用。最后,











































 京公网安备 11010802027423号
京公网安备 11010802027423号