当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Expression, Purification, and Biochemical Characterization of Human Afamin.
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2018-02-20 , DOI: 10.1021/acs.jproteome.7b00867 Alessandra Altamirano , Andreas Naschberger , Barbara G Fürnrohr , Radka Saldova 1 , Weston B Struwe 1 , Patrick M Jennings 1 , Silvia Millán Martín 1 , Suzana Malic 2 , Immanuel Plangger , Stefan Lechner , Reina Pisano , Nicole Peretti , Bernd Linke , Mario M Aguiar , Friedrich Fresser , Andreas Ritsch , Tihana Lenac Rovis 2 , Christina Goode , Pauline M Rudd 1 , Klaus Scheffzek , Bernhard Rupp , Hans Dieplinger 3
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2018-02-20 , DOI: 10.1021/acs.jproteome.7b00867 Alessandra Altamirano , Andreas Naschberger , Barbara G Fürnrohr , Radka Saldova 1 , Weston B Struwe 1 , Patrick M Jennings 1 , Silvia Millán Martín 1 , Suzana Malic 2 , Immanuel Plangger , Stefan Lechner , Reina Pisano , Nicole Peretti , Bernd Linke , Mario M Aguiar , Friedrich Fresser , Andreas Ritsch , Tihana Lenac Rovis 2 , Christina Goode , Pauline M Rudd 1 , Klaus Scheffzek , Bernhard Rupp , Hans Dieplinger 3
Affiliation
|
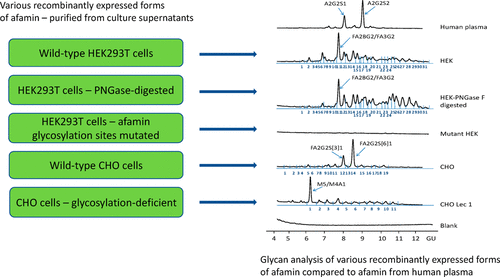
Afamin is an 87 kDa glycoprotein with five predicted N-glycosylation sites. Afamin's glycan abundance contributes to conformational and chemical inhomogeneity presenting great challenges for molecular structure determination. For the purpose of studying the structure of afamin, various forms of recombinantly expressed human afamin (rhAFM) with different glycosylation patterns were thus created. Wild-type rhAFM and various hypoglycosylated forms were expressed in CHO, CHO-Lec1, and HEK293T cells. Fully nonglycosylated rhAFM was obtained by transfection of point-mutated cDNA to delete all N-glycosylation sites of afamin. Wild-type and hypo/nonglycosylated rhAFM were purified from cell culture supernatants by immobilized metal ion affinity and size exclusion chromatography. Glycan analysis of purified proteins demonstrated differences in micro- and macro-heterogeneity of glycosylation enabling the comparison between hypoglycosylated, wild-type rhAFM, and native plasma afamin. Because antibody fragments can work as artificial chaperones by stabilizing the structure of proteins and consequently enhance the chance for successful crystallization, we incubated a Fab fragment of the monoclonal anti-afamin antibody N14 with human afamin and obtained a stoichiometric complex. Subsequent results showed sufficient expression of various partially or nonglycosylated forms of rhAFM in HEK293T and CHO cells and revealed that glycosylation is not necessary for expression and secretion.
中文翻译:

人Afamin的表达,纯化和生化特性。
Afamin是一个87 kDa的糖蛋白,具有5个预测的N-糖基化位点。Afamin的聚糖丰富度导致构象和化学不均一性,对分子结构测定提出了巨大挑战。为了研究Afamin的结构,因此创建了具有不同糖基化模式的多种形式的重组表达的人Afamin(rhAFM)。野生型rhAFM和各种低糖基化形式在CHO,CHO-Lec1和HEK293T细胞中表达。通过转染点突变的cDNA删除afamin的所有N-糖基化位点,可以得到完全非糖基化的rhAFM。通过固定的金属离子亲和力和尺寸排阻色谱法从细胞培养上清液中纯化野生型和低/非糖基化的rhAFM。对纯化蛋白进行的糖分析表明,糖基化在微观和宏观异质性方面存在差异,从而可以比较低糖基化,野生型rhAFM和天然血浆afamin。由于抗体片段可以通过稳定蛋白质的结构而充当人工伴侣,因此增加了成功结晶的机会,因此我们将单克隆抗-Afamin抗体N14的Fab片段与人Afamin进行了温育,从而获得了化学计量的配合物。随后的结果表明,rHEAFM的各种部分或非糖基化形式的rhAFM在HEK293T和CHO细胞中充分表达,并表明糖基化对于表达和分泌不是必需的。由于抗体片段可以通过稳定蛋白质的结构而充当人工伴侣,因此增加了成功结晶的机会,因此我们将单克隆抗-Afamin抗体N14的Fab片段与人Afamin进行了温育,从而获得了化学计量的配合物。随后的结果表明,rHEAFM的各种部分或非糖基化形式的rhAFM在HEK293T和CHO细胞中充分表达,并表明糖基化对于表达和分泌不是必需的。由于抗体片段可以通过稳定蛋白质的结构而充当人工伴侣,因此增加了成功结晶的机会,因此我们将单克隆抗-Afamin抗体N14的Fab片段与人Afamin进行了温育,从而获得了化学计量的配合物。随后的结果表明,rHEAFM的各种部分或非糖基化形式的rhAFM在HEK293T和CHO细胞中充分表达,并表明糖基化对于表达和分泌不是必需的。
更新日期:2018-02-21
中文翻译:

人Afamin的表达,纯化和生化特性。
Afamin是一个87 kDa的糖蛋白,具有5个预测的N-糖基化位点。Afamin的聚糖丰富度导致构象和化学不均一性,对分子结构测定提出了巨大挑战。为了研究Afamin的结构,因此创建了具有不同糖基化模式的多种形式的重组表达的人Afamin(rhAFM)。野生型rhAFM和各种低糖基化形式在CHO,CHO-Lec1和HEK293T细胞中表达。通过转染点突变的cDNA删除afamin的所有N-糖基化位点,可以得到完全非糖基化的rhAFM。通过固定的金属离子亲和力和尺寸排阻色谱法从细胞培养上清液中纯化野生型和低/非糖基化的rhAFM。对纯化蛋白进行的糖分析表明,糖基化在微观和宏观异质性方面存在差异,从而可以比较低糖基化,野生型rhAFM和天然血浆afamin。由于抗体片段可以通过稳定蛋白质的结构而充当人工伴侣,因此增加了成功结晶的机会,因此我们将单克隆抗-Afamin抗体N14的Fab片段与人Afamin进行了温育,从而获得了化学计量的配合物。随后的结果表明,rHEAFM的各种部分或非糖基化形式的rhAFM在HEK293T和CHO细胞中充分表达,并表明糖基化对于表达和分泌不是必需的。由于抗体片段可以通过稳定蛋白质的结构而充当人工伴侣,因此增加了成功结晶的机会,因此我们将单克隆抗-Afamin抗体N14的Fab片段与人Afamin进行了温育,从而获得了化学计量的配合物。随后的结果表明,rHEAFM的各种部分或非糖基化形式的rhAFM在HEK293T和CHO细胞中充分表达,并表明糖基化对于表达和分泌不是必需的。由于抗体片段可以通过稳定蛋白质的结构而充当人工伴侣,因此增加了成功结晶的机会,因此我们将单克隆抗-Afamin抗体N14的Fab片段与人Afamin进行了温育,从而获得了化学计量的配合物。随后的结果表明,rHEAFM的各种部分或非糖基化形式的rhAFM在HEK293T和CHO细胞中充分表达,并表明糖基化对于表达和分泌不是必需的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号