Journal of Controlled Release ( IF 10.5 ) Pub Date : 2018-02-14 , DOI: 10.1016/j.jconrel.2018.02.014 Yi Shu 1 , Hongran Yin 2 , Mehdi Rajabi 1 , Hui Li 3 , Mario Vieweger 2 , Sijin Guo 2 , Dan Shu 2 , Peixuan Guo 2
|
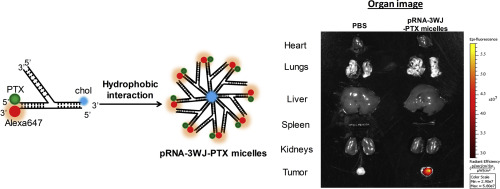
RNA can serve as powerful building blocks for bottom-up fabrication of nanostructures for biotechnological and biomedical applications. In addition to current self-assembly strategies utilizing base pairing, motif piling and tertiary interactions, we reported for the first time the formation of RNA based micellar nanoconstruct with a cholesterol molecule conjugated onto one helical end of a branched pRNA three-way junction (3WJ) motif. The resulting amphiphilic RNA micelles consist of a hydrophilic RNA head and a covalently linked hydrophobic lipid tail that can spontaneously assemble in aqueous solution via hydrophobic interaction. Taking advantage of pRNA 3WJ branched structure, the assembled RNA micelles are capable of escorting multiple functional modules. As a proof of concept for delivery for therapeutics, Paclitaxel was loaded into the RNA micelles with significantly improved water solubility. The successful construction of the drug loaded RNA micelles was confirmed and characterized by agarose gel electrophoresis, atomic force microscopy (AFM), dynamic light scattering (DLS), and fluorescence Nile Red encapsulation assay. The estimate critical micelle formation concentration ranges from 39 nM to 78 nM. The Paclitaxel loaded RNA micelles can internalize into cancer cells and inhibit their proliferation. Further studies showed that the Paclitaxel loaded RNA micelles induced cancer cell apoptosis in a Caspase-3 dependent manner but RNA micelles alone exhibited low cytotoxicity. Finally, the Paclitaxel loaded RNA micelles targeted to tumor in vivo without accumulation in healthy tissues and organs. There is also no or very low induction of pro-inflammatory response. Therefore, multivalence, cancer cell permeability, combined with controllable assembly, low or non toxic nature, and tumor targeting are all promising features that make our pRNA micelles a suitable platform for potential drug delivery.
中文翻译:

基于 RNA 的胶束:紫杉醇装载和递送的新型平台
RNA 可以作为生物技术和生物医学应用中自下而上制造纳米结构的强大构建模块。除了目前利用碱基配对、基序堆积和三级相互作用的自组装策略外,我们还首次报道了基于 RNA 的胶束纳米结构的形成,其中胆固醇分子缀合到分支 pRNA 三向连接的一个螺旋末端 (3WJ )主题。由此产生的两亲性 RNA 胶束由亲水性 RNA 头部和共价连接的疏水性脂质尾部组成,可以通过疏水相互作用在水溶液中自发组装。利用pRNA 3WJ分支结构,组装的RNA胶束能够护送多个功能模块。作为治疗传递概念的证明,紫杉醇被加载到 RNA 胶束中,水溶性显着提高。通过琼脂糖凝胶电泳、原子力显微镜(AFM)、动态光散射(DLS)和荧光尼罗红封装测定证实和表征了载药RNA胶束的成功构建。估计的临界胶束形成浓度范围为 39 nM 至 78 nM。负载紫杉醇的 RNA 胶束可以内化到癌细胞中并抑制其增殖。进一步的研究表明,负载紫杉醇的RNA胶束以Caspase-3依赖性方式诱导癌细胞凋亡,但单独的RNA胶束表现出较低的细胞毒性。最后,紫杉醇负载的RNA胶束在体内靶向肿瘤,而不会在健康组织和器官中积累。也没有或非常低地诱导促炎反应。因此,多价、癌细胞渗透性、与可控组装、低毒或无毒性质以及肿瘤靶向性相结合,这些都是有前途的特征,使我们的 pRNA 胶束成为潜在药物输送的合适平台。










































 京公网安备 11010802027423号
京公网安备 11010802027423号