当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Forty Years after “Heterodiene Syntheses with α,β-Unsaturated Carbonyl Compounds”: Enantioselective Syntheses of 3,4-Dihydropyran Derivatives
Chemical Reviews ( IF 51.4 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1021/acs.chemrev.7b00322 Giovanni Desimoni 1 , Giuseppe Faita 1 , Paolo Quadrelli 1
Chemical Reviews ( IF 51.4 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1021/acs.chemrev.7b00322 Giovanni Desimoni 1 , Giuseppe Faita 1 , Paolo Quadrelli 1
Affiliation
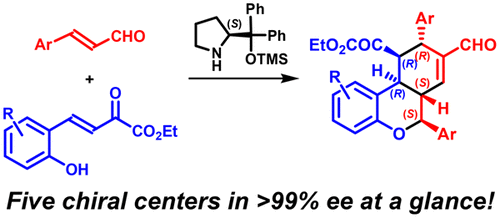
|
This review is focused on the enantioselective synthesis of 3,4-dihydropyran derivatives, whose importance as chiral building blocks in the synthesis of bioactive molecules and natural products is well established. The review analyzes the different synthetic strategies by grouping them as a function of the atom numbers of the reagents involved. Starting from the classical [4 + 2] and [2 + 4] approaches, the [3 + 3], [5 + 1], and [6] strategies have been sequentially analyzed, and for each of them, the asymmetry induced by both chiral metal complexes and different kinds of organocatalysts has been examined. More than 400 papers have been reviewed, whose results have been described in the highest synthetic manner, in the attempt to emphasize the mechanism of the chirality transfer from the chiral messengers to the reaction products. This analysis allows the great flexibility of the diverse catalytic systems, the complementary of the results obtained from the different reaction pathways, and the very high level of control of the achievable molecular complexity to be evidenced.
中文翻译:

“由α,β-不饱和羰基化合物杂二烯合成”后的四十年:3,4-二氢吡喃衍生物的对映选择性合成
这篇综述集中在3,4-二氢吡喃衍生物的对映选择性合成上,其在生物活性分子和天然产物的合成中作为手性结构单元的重要性已得到充分确立。该综述通过将不同的合成策略根据所涉及试剂的原子序数进行分组来进行分析。从经典的[4 + 2]和[2 + 4]方法开始,已经依次分析了[3 + 3],[5 +1]和[6]策略,并且对于每种策略,由手性金属络合物和不同种类的有机催化剂均已进行了研究。为了强调从手性信使剂向反应产物的手性转移的机理,已经综述了400多篇论文,其结果以最高的合成方式进行了描述。
更新日期:2018-02-14
中文翻译:

“由α,β-不饱和羰基化合物杂二烯合成”后的四十年:3,4-二氢吡喃衍生物的对映选择性合成
这篇综述集中在3,4-二氢吡喃衍生物的对映选择性合成上,其在生物活性分子和天然产物的合成中作为手性结构单元的重要性已得到充分确立。该综述通过将不同的合成策略根据所涉及试剂的原子序数进行分组来进行分析。从经典的[4 + 2]和[2 + 4]方法开始,已经依次分析了[3 + 3],[5 +1]和[6]策略,并且对于每种策略,由手性金属络合物和不同种类的有机催化剂均已进行了研究。为了强调从手性信使剂向反应产物的手性转移的机理,已经综述了400多篇论文,其结果以最高的合成方式进行了描述。









































 京公网安备 11010802027423号
京公网安备 11010802027423号