当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Encapsulation of Ionic Liquids with an Aprotic Heterocyclic Anion (AHA-IL) for CO2 Capture: Preserving the Favorable Thermodynamics and Enhancing the Kinetics of Absorption
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1021/acs.jpcb.7b12137 Cristian Moya 1 , Noelia Alonso-Morales 1 , Juan de Riva 1 , Oscar Morales-Collazo 2 , Joan F. Brennecke 2 , Jose Palomar 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1021/acs.jpcb.7b12137 Cristian Moya 1 , Noelia Alonso-Morales 1 , Juan de Riva 1 , Oscar Morales-Collazo 2 , Joan F. Brennecke 2 , Jose Palomar 1
Affiliation
|
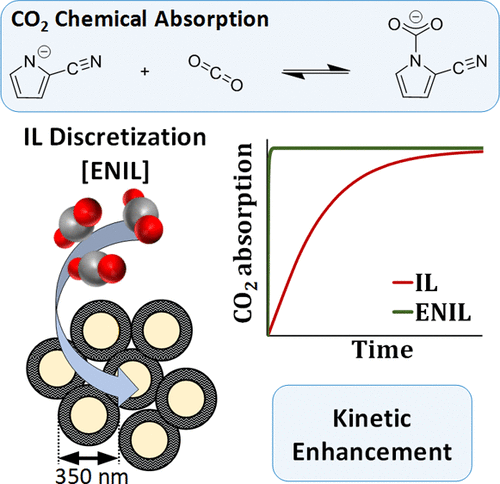
The performance of an ionic liquid with an aprotic heterocyclic anion (AHA-IL), trihexyl(tetradecyl)phosphonium 2-cyanopyrrolide ([P66614][2-CNPyr]), for CO2 capture has been evaluated considering both the thermodynamics and the kinetics of the phenomena. Absorption gravimetric measurements of the gas–liquid equilibrium isotherms of CO2–AHA-IL systems were carried out from 298 to 333 K and at pressures up to 15 bar, analyzing the role of both chemical and physical absorption phenomena in the overall CO2 solubility in the AHA-IL, as has been done previously. In addition, the kinetics of the CO2 chemical absorption process was evaluated by in situ Fourier transform infrared spectroscopy-attenuated total reflection, following the characteristic vibrational signals of the reactants and products over the reaction time. A chemical absorption model was used to describe the time-dependent concentration of species involved in the reactive absorption, obtaining kinetic parameters (such as chemical reaction kinetic constants and diffusion coefficients) as a function of temperatures and pressures. As expected, the results demonstrate that the CO2 absorption rate is mass-transfer-controlled because of the relatively high viscosity of AHA-IL. The AHA-IL was encapsulated in a porous carbon sphere (Encapsulated Ionic Liquid, ENIL) to improve the kinetic performance of the AHA-IL for CO2 capture. The newly synthesized AHA-ENIL material was evaluated as a CO2 sorbent with gravimetric absorption measurements. AHA-ENIL systems preserve the good CO2 absorption capacity of the AHA-IL but drastically enhance the CO2 absorption rate because of the increased gas–liquid surface contact area achieved by solvent encapsulation.
中文翻译:

离子液体与非质子杂环阴离子(AHA-IL)的结合,用于CO 2捕集:保留有利的热力学并增强吸收动力学
评估了具有质子惰性杂环阴离子(AHA-IL),三己基(十四烷基)phosph 2-氰基吡咯化物([P 66614 ] [2-CNPyr])的离子液体的CO 2捕集性能,同时考虑了热力学和热力学。现象的动力学。在298至333 K且压力高达15 bar的条件下,对CO 2 -AHA-IL系统的气液平衡等温线进行吸收重量分析,分析了化学吸收现象和物理吸收现象在整个CO 2溶解度中的作用像以前一样在AHA-IL中进行操作。此外,CO 2的动力学通过在反应时间内反应物和产物的特征振动信号,通过傅里叶变换红外光谱衰减全反射来评估化学吸收过程。使用化学吸收模型来描述反应吸收中所涉及的物质的时间依赖性浓度,从而获得随温度和压力变化的动力学参数(例如化学反应动力学常数和扩散系数)。如所预期的,结果表明,由于AHA-IL的相对较高的粘度,因此CO 2吸收速率受传质控制。将AHA-IL封装在多孔碳球(Encapsulated Ionic Liquid,ENIL)中,以改善AHA-IL对CO 2的动力学性能。捕获。通过重量吸收测量,将新合成的AHA-ENIL材料评估为CO 2吸附剂。AHA-ENIL系统保留了AHA-IL良好的CO 2吸收能力,但由于溶剂封装增加了气液表面接触面积,因此大大提高了CO 2吸收率。
更新日期:2018-02-14
中文翻译:

离子液体与非质子杂环阴离子(AHA-IL)的结合,用于CO 2捕集:保留有利的热力学并增强吸收动力学
评估了具有质子惰性杂环阴离子(AHA-IL),三己基(十四烷基)phosph 2-氰基吡咯化物([P 66614 ] [2-CNPyr])的离子液体的CO 2捕集性能,同时考虑了热力学和热力学。现象的动力学。在298至333 K且压力高达15 bar的条件下,对CO 2 -AHA-IL系统的气液平衡等温线进行吸收重量分析,分析了化学吸收现象和物理吸收现象在整个CO 2溶解度中的作用像以前一样在AHA-IL中进行操作。此外,CO 2的动力学通过在反应时间内反应物和产物的特征振动信号,通过傅里叶变换红外光谱衰减全反射来评估化学吸收过程。使用化学吸收模型来描述反应吸收中所涉及的物质的时间依赖性浓度,从而获得随温度和压力变化的动力学参数(例如化学反应动力学常数和扩散系数)。如所预期的,结果表明,由于AHA-IL的相对较高的粘度,因此CO 2吸收速率受传质控制。将AHA-IL封装在多孔碳球(Encapsulated Ionic Liquid,ENIL)中,以改善AHA-IL对CO 2的动力学性能。捕获。通过重量吸收测量,将新合成的AHA-ENIL材料评估为CO 2吸附剂。AHA-ENIL系统保留了AHA-IL良好的CO 2吸收能力,但由于溶剂封装增加了气液表面接触面积,因此大大提高了CO 2吸收率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号