当前位置:
X-MOL 学术
›
Mol. Syst. Des. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Water dissolution in ionic liquids between charged surfaces: effects of electric polarization and electrostatic correlation†
Molecular Systems Design & Engineering ( IF 3.2 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1039/c7me00119c Hongbo Chen 1, 2, 3, 4, 5 , Lijia An 1, 2, 3, 4, 5 , Issei Nakamura 6, 7, 8, 9
Molecular Systems Design & Engineering ( IF 3.2 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1039/c7me00119c Hongbo Chen 1, 2, 3, 4, 5 , Lijia An 1, 2, 3, 4, 5 , Issei Nakamura 6, 7, 8, 9
Affiliation
|
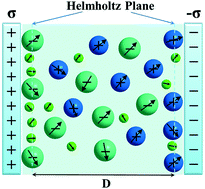
Water dissolved in ionic liquids garners particular attention in electrochemistry, as represented by the case where water molecules cannot be completely removed from ionic liquids. Nevertheless, the effects of such polarizable polar molecules on the energy efficiency of electrochemical devices remain elusive. Thus, we highlight the effects of the spatially varying dielectric response of water and ionic liquid near charged plates using a coarse-grained mean-field theory that simultaneously accounts for both the permanent and induced dipole moments of the species and the strong electrostatic correlation. We show that water can be adsorbed onto electrodes primarily because of the dielectric contrast between the species. Our results predict that linear-dielectric theory is inadequate to account for the correlation between the capacitance and dielectric contrast, which may be fitted by exponential functions. Electronic polarizability can enhance the capacitance. A higher-dielectric component preferentially solvates electrodes, but this effect competes with that of the charge screening. Our theory shows that strong electrostatic correlation causes the dipolar cation and anion to form alternating layers, which in turn yields substantial increases in the capacitance. Our results compare favorably with previous molecular dynamics simulations.
中文翻译:

带电表面之间的离子液体中的水溶解度:电极化和静电相关的影响†
溶解在离子液体中的水在电化学领域尤其受关注,如无法从离子液体中完全除去水分子的情况所代表的那样。然而,这种可极化的极性分子对电化学装置的能量效率的影响仍然难以捉摸。因此,我们使用粗糙的平均场理论,强调了带电板附近水和离子液体在空间上变化的介电响应的影响,该理论同时考虑了物种的永久偶极矩和感应偶极矩以及强静电相关性。我们表明水可以被吸附到电极上,主要是由于物质之间的介电对比。我们的结果预测,线性介电理论不足以解决电容和介电对比度之间的相关性,这可以通过指数函数来拟合。电子极化率可以增强电容。高介电常数的组分优先溶剂化电极,但是这种效果与电荷屏蔽的效果不相上下。我们的理论表明,强的静电相关性会导致偶极阳离子和阴离子形成交替的层,进而导致电容的显着增加。我们的结果与以前的分子动力学模拟相比具有优势。但是这种效果与电荷筛选的效果不相上下。我们的理论表明,强的静电相关性会导致偶极阳离子和阴离子形成交替的层,进而导致电容的显着增加。我们的结果与以前的分子动力学模拟相比具有优势。但是这种效果与电荷筛选的效果不相上下。我们的理论表明,强的静电相关性会导致偶极阳离子和阴离子形成交替的层,进而导致电容的显着增加。我们的结果与以前的分子动力学模拟相比具有优势。
更新日期:2018-02-14
中文翻译:

带电表面之间的离子液体中的水溶解度:电极化和静电相关的影响†
溶解在离子液体中的水在电化学领域尤其受关注,如无法从离子液体中完全除去水分子的情况所代表的那样。然而,这种可极化的极性分子对电化学装置的能量效率的影响仍然难以捉摸。因此,我们使用粗糙的平均场理论,强调了带电板附近水和离子液体在空间上变化的介电响应的影响,该理论同时考虑了物种的永久偶极矩和感应偶极矩以及强静电相关性。我们表明水可以被吸附到电极上,主要是由于物质之间的介电对比。我们的结果预测,线性介电理论不足以解决电容和介电对比度之间的相关性,这可以通过指数函数来拟合。电子极化率可以增强电容。高介电常数的组分优先溶剂化电极,但是这种效果与电荷屏蔽的效果不相上下。我们的理论表明,强的静电相关性会导致偶极阳离子和阴离子形成交替的层,进而导致电容的显着增加。我们的结果与以前的分子动力学模拟相比具有优势。但是这种效果与电荷筛选的效果不相上下。我们的理论表明,强的静电相关性会导致偶极阳离子和阴离子形成交替的层,进而导致电容的显着增加。我们的结果与以前的分子动力学模拟相比具有优势。但是这种效果与电荷筛选的效果不相上下。我们的理论表明,强的静电相关性会导致偶极阳离子和阴离子形成交替的层,进而导致电容的显着增加。我们的结果与以前的分子动力学模拟相比具有优势。











































 京公网安备 11010802027423号
京公网安备 11010802027423号