当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sub-15 nm CeO2 nanowires as an efficient non-noble metal catalyst in the room-temperature oxidation of aniline†
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1039/c7cy02402a Anderson G. M. da Silva 1, 2, 3, 4, 5 , Daniel C. Batalha 5, 6, 7 , Thenner S. Rodrigues 4, 5, 8, 9 , Eduardo G. Candido 1, 2, 3, 4, 5 , Sulusmon C. Luz 5, 6, 7 , Isabel C. de Freitas 1, 2, 3, 4, 5 , Fabio C. Fonseca 4, 5, 8, 9 , Daniela C. de Oliveira 5, 10, 11, 12 , Jason G. Taylor 5, 6, 7 , Susana I. Córdoba de Torresi 1, 2, 3, 4, 5 , Pedro H. C. Camargo 1, 2, 3, 4, 5 , Humberto V. Fajardo 5, 6, 7
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1039/c7cy02402a Anderson G. M. da Silva 1, 2, 3, 4, 5 , Daniel C. Batalha 5, 6, 7 , Thenner S. Rodrigues 4, 5, 8, 9 , Eduardo G. Candido 1, 2, 3, 4, 5 , Sulusmon C. Luz 5, 6, 7 , Isabel C. de Freitas 1, 2, 3, 4, 5 , Fabio C. Fonseca 4, 5, 8, 9 , Daniela C. de Oliveira 5, 10, 11, 12 , Jason G. Taylor 5, 6, 7 , Susana I. Córdoba de Torresi 1, 2, 3, 4, 5 , Pedro H. C. Camargo 1, 2, 3, 4, 5 , Humberto V. Fajardo 5, 6, 7
Affiliation
|
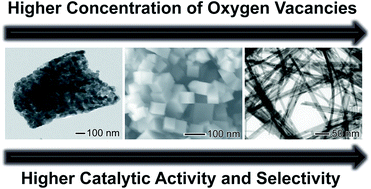
We described herein the facile synthesis of sub-15 nm CeO2 nanowires based on a hydrothermal method without the use of any capping/stabilizing agent, in which an oriented attachment mechanism took place during the CeO2 nanowire formation. The synthesis of sub-15 nm CeO2 nanowires could be achieved on relatively large scales (∼2.6 grams of nanowires per batch), in high yields (>94%), and at low cost. To date, there are only a limited number of successful attempts towards the synthesis of CeO2 nanowires with such small diameters, and the reported protocols are typically limited to low amounts. The nanowires displayed uniform shapes and sizes, high surface areas, an increased number of oxygen defects sites, and a high proportion of Ce3+/Ce4+ surface species. These features make them promising candidates for oxidation reactions. To this end, we employed the selective oxidation of aniline as a model transformation. The sub-15 nm CeO2 nanowires catalyzed the selective synthesis of nitrosobenzene (up to 98% selectivity) from aniline at room temperature using H2O2 as the oxidant. The effect of solvent and temperature during the catalytic reaction was investigated. We found that such parameters played an important role in the control of the selectivity. The improved catalytic activities observed for the sub-15 nm nanowires could be explained by: i) the uniform morphology with a typical dimension of 11 ± 2 nm in width, which provides higher specific surface areas relative to those of conventional catalysts; ii) the significant concentration of oxygen vacancies and high proportion of Ce3+/Ce4+ species at the surface that represent highly active sites towards oxidation reactions; iii) the crystal growth along the (110) highly catalytically active crystallographic directions, and iv) the mesoporous surface which is easily accessible by liquid substrates. The results reported herein demonstrated high activities under ambient conditions, provided novel insights into selectivities, and may inspire novel metal oxide-based catalysts with desired performances.
中文翻译:

亚15纳米CeO 2纳米线可在室温下苯胺的氧化中作为有效的非贵金属催化剂†
我们在本文中描述了基于水热方法的亚15 nm CeO 2纳米线的简便合成,而无需使用任何封端/稳定剂,其中在CeO 2纳米线形成过程中发生了定向附着机制。低于15 nm的CeO 2纳米线的合成可以以相对较高的规模(每批约2.6克纳米线),高产率(> 94%)和低成本实现。迄今为止,只有很少的成功尝试来合成具有如此小直径的CeO 2纳米线,并且所报道的方案通常限于少量。纳米线显示出均匀的形状和大小,高的表面积,增加的氧缺陷位点数量以及高比例的铈3+ / Ce 4+表面物种。这些特征使其成为氧化反应的有希望的候选者。为此,我们采用了苯胺的选择性氧化作为模型转化。低于15 nm的CeO 2纳米线在室温下使用H 2 O 2催化从苯胺选择性合成亚硝基苯(选择性高达98%)。作为氧化剂。研究了溶剂和温度对催化反应的影响。我们发现这些参数在选择性的控制中起着重要的作用。对于亚15纳米线观察到的改善的催化活性可以通过以下方式解释:i)均匀的形态,典型的宽度为11±2 nm,与常规催化剂相比具有更高的比表面积;ii)明显的氧空位浓度和高比例的Ce 3+ / Ce 4+表面上的物种代表着对氧化反应具有高活性的位点;iii)沿(110)高催化活性晶体学方向的晶体生长,以及iv)液体基质易于接近的中孔表面。本文报道的结果证明了在环境条件下的高活性,提供了对选择性的新颖见解,并可以启发具有所需性能的新型基于金属氧化物的催化剂。
更新日期:2018-02-14
中文翻译:

亚15纳米CeO 2纳米线可在室温下苯胺的氧化中作为有效的非贵金属催化剂†
我们在本文中描述了基于水热方法的亚15 nm CeO 2纳米线的简便合成,而无需使用任何封端/稳定剂,其中在CeO 2纳米线形成过程中发生了定向附着机制。低于15 nm的CeO 2纳米线的合成可以以相对较高的规模(每批约2.6克纳米线),高产率(> 94%)和低成本实现。迄今为止,只有很少的成功尝试来合成具有如此小直径的CeO 2纳米线,并且所报道的方案通常限于少量。纳米线显示出均匀的形状和大小,高的表面积,增加的氧缺陷位点数量以及高比例的铈3+ / Ce 4+表面物种。这些特征使其成为氧化反应的有希望的候选者。为此,我们采用了苯胺的选择性氧化作为模型转化。低于15 nm的CeO 2纳米线在室温下使用H 2 O 2催化从苯胺选择性合成亚硝基苯(选择性高达98%)。作为氧化剂。研究了溶剂和温度对催化反应的影响。我们发现这些参数在选择性的控制中起着重要的作用。对于亚15纳米线观察到的改善的催化活性可以通过以下方式解释:i)均匀的形态,典型的宽度为11±2 nm,与常规催化剂相比具有更高的比表面积;ii)明显的氧空位浓度和高比例的Ce 3+ / Ce 4+表面上的物种代表着对氧化反应具有高活性的位点;iii)沿(110)高催化活性晶体学方向的晶体生长,以及iv)液体基质易于接近的中孔表面。本文报道的结果证明了在环境条件下的高活性,提供了对选择性的新颖见解,并可以启发具有所需性能的新型基于金属氧化物的催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号