当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct catalytic enantioselective amination of ketones for the formation of tri- and tetrasubstituted stereocenters†
Chemical Science ( IF 7.6 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1039/c8sc00147b B M Trost 1 , J S Tracy 1 , T Saget 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1039/c8sc00147b B M Trost 1 , J S Tracy 1 , T Saget 1
Affiliation
|
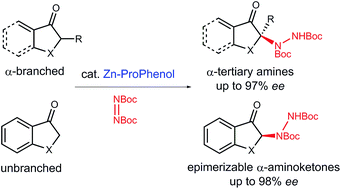
Herein, we report a Zn–ProPhenol catalyzed direct asymmetric amination reaction of unactivated aryl and vinyl ketones using di-tert-butyl azodicarboxylate as a cheap and practical electrophilic nitrogen source. Importantly, this methodology works with both α-branched and unbranched ketones for the construction of tri- and tetrasubstituted N-containing stereocenters. The reaction can be run at gram-scale with low catalyst loadings and features a recoverable and reusable ligand. Finally, the enantioenriched hydrazine products can be readily converted into versatile building blocks such as α-amino carbonyl compounds and β-amino alcohols.
中文翻译:

酮的直接催化对映选择性胺化以形成三取代和四取代的立体中心†
在此,我们报道了使用偶氮二甲酸二叔丁酯作为廉价实用的亲电氮源,Zn-ProPhenol 催化未活化的芳基和乙烯基酮的直接不对称胺化反应。重要的是,该方法适用于 α-支化和非支化酮,用于构建三取代和四取代的含氮立构中心。该反应可以在克级规模、低催化剂负载量下进行,并具有可回收和可重复使用的配体。最后,对映体富集的肼产品可以很容易地转化为多功能结构单元,例如α-氨基羰基化合物和β-氨基醇。
更新日期:2018-02-14
中文翻译:

酮的直接催化对映选择性胺化以形成三取代和四取代的立体中心†
在此,我们报道了使用偶氮二甲酸二叔丁酯作为廉价实用的亲电氮源,Zn-ProPhenol 催化未活化的芳基和乙烯基酮的直接不对称胺化反应。重要的是,该方法适用于 α-支化和非支化酮,用于构建三取代和四取代的含氮立构中心。该反应可以在克级规模、低催化剂负载量下进行,并具有可回收和可重复使用的配体。最后,对映体富集的肼产品可以很容易地转化为多功能结构单元,例如α-氨基羰基化合物和β-氨基醇。











































 京公网安备 11010802027423号
京公网安备 11010802027423号