当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A "Tug of War" Maintains a Dynamic Protein-Membrane Complex: Molecular Dynamics Simulations of C-Raf RBD-CRD Bound to K-Ras4B at an Anionic Membrane.
ACS Central Science ( IF 12.7 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1021/acscentsci.7b00593 Zhen-Lu Li 1 , Priyanka Prakash 2 , Matthias Buck 1, 1, 1, 1, 1
ACS Central Science ( IF 12.7 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1021/acscentsci.7b00593 Zhen-Lu Li 1 , Priyanka Prakash 2 , Matthias Buck 1, 1, 1, 1, 1
Affiliation
|
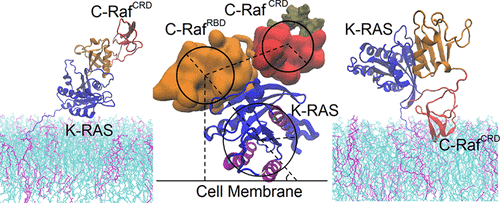
Association of Raf kinase with activated Ras triggers downstream signaling cascades toward regulating transcription in the cells’ nucleus. Dysregulation of Ras–Raf signaling stimulates cancers. We investigate the C-Raf RBD and CRD regions when bound to oncogenic K-Ras4B at the membrane. All-atom molecular dynamics simulations suggest that the membrane plays an integral role in regulating the configurational ensemble of the complex. Remarkably, the complex samples a few states dynamically, reflecting a competition between C-Raf CRD- and K-Ras4B- membrane interactions. This competition arises because the interaction between the RBD and K-Ras is strong while the linker between the RBD and CRD is short. Such a mechanism maintains a modest binding for the overall complex at the membrane and is expected to facilitate fast signaling processes. Competition of protein–membrane contacts is likely a common mechanism for other multiprotein complexes, if not multidomain proteins at membranes.
中文翻译:

“拔河比赛”维持着动态的蛋白质膜复合物:在阴离子膜上与K-Ras4B结合的C-Raf RBD-CRD的分子动力学模拟。
Raf激酶与激活的Ras的结合会触发下游信号传导级联,以调节细胞核中的转录。Ras–Raf信号传导异常会刺激癌症。当在膜上与致癌的K-Ras4B结合时,我们研究了C-Raf RBD和CRD区。全原子分子动力学模拟表明,膜在调节复合物的构型整体中起着不可或缺的作用。值得注意的是,该复合物动态地采样了一些状态,反映了C-Raf CRD-和K-Ras4B-膜相互作用之间的竞争。之所以出现这种竞争,是因为RBD与K-Ras之间的相互作用很强,而RBD与CRD之间的连接子却很短。这种机制对膜上的整个复合物保持适度的结合,并有望促进快速的信号传导过程。
更新日期:2018-02-14
中文翻译:

“拔河比赛”维持着动态的蛋白质膜复合物:在阴离子膜上与K-Ras4B结合的C-Raf RBD-CRD的分子动力学模拟。
Raf激酶与激活的Ras的结合会触发下游信号传导级联,以调节细胞核中的转录。Ras–Raf信号传导异常会刺激癌症。当在膜上与致癌的K-Ras4B结合时,我们研究了C-Raf RBD和CRD区。全原子分子动力学模拟表明,膜在调节复合物的构型整体中起着不可或缺的作用。值得注意的是,该复合物动态地采样了一些状态,反映了C-Raf CRD-和K-Ras4B-膜相互作用之间的竞争。之所以出现这种竞争,是因为RBD与K-Ras之间的相互作用很强,而RBD与CRD之间的连接子却很短。这种机制对膜上的整个复合物保持适度的结合,并有望促进快速的信号传导过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号