当前位置:
X-MOL 学术
›
Ceram. Int.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Magnesium ferrite nanoparticles as a magnetic sorbent for the removal of Mn 2+ , Co 2+ , Ni 2+ and Cu 2+ from aqueous solution
Ceramics International ( IF 5.2 ) Pub Date : 2018-06-01 , DOI: 10.1016/j.ceramint.2018.02.117 A.I. Ivanets , V. Srivastava , M.Yu. Roshchina , M. Sillanpää , V.G. Prozorovich , V.V. Pankov
Ceramics International ( IF 5.2 ) Pub Date : 2018-06-01 , DOI: 10.1016/j.ceramint.2018.02.117 A.I. Ivanets , V. Srivastava , M.Yu. Roshchina , M. Sillanpää , V.G. Prozorovich , V.V. Pankov
|
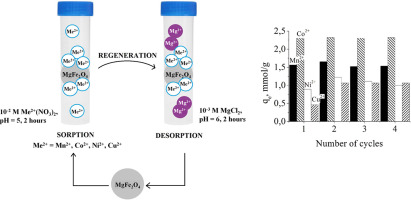
Abstract The aim of this research was to prepare magnesium ferrite (MgFe2O4) magnetic nanoparticles and to investigate their sorption characteristics towards Mn2+, Co2+, Ni2+, Cu2+ ions in aqueous solution. MgFe2O4 was synthesized by glycine-nitrate combustion method and was characterized by low crystallinity with crystallite size of 8.2 nm, particle aggregates of 13–25 nm, BET surface area of 14 m2/g and pore size of 8.0 nm. Sorption properties of MgFe2O4 towards Mn2+, Co2+, Ni2+, Cu2+ ions were studied using one-component model solutions and found to be dependent on metal ions concentration, contact time, pH and conditions of regeneration experiment. The highest sorption capacity of MgFe2O4 was detected towards Co2+ (2.30 mmol g1) and Mn2+ (1.56 mmol g−1) and the lowest towards Ni2+ (0.89 mmol g−1) and Cu2+ (0.46 mmol g−1). It was observed that sorption equilibrium occurs very quickly within 20–60 min. The pHzpc of sorbent was calculated to be 6.58. At studied pH interval (3.0–7.0) the sorption capacity of MgFe2O4 was not significantly affected. Regeneration study showed that the metal loaded sorbent could be regenerated by aqueous solution of 10−3 M MgCl2 at pH 6.0 within 120 min of contact time. Regeneration test suggested that MgFe2O4 magnetic sorbent can be efficiently used at least for four adsorption-desorption cycles. The high sorption properties and kinetics of toxic metal ion sorption indicates good prospects of developed sorbent in practice for wastewater treatment.
中文翻译:

铁氧体镁纳米颗粒作为磁性吸附剂,用于从水溶液中去除 Mn 2+ 、Co 2+ 、Ni 2+ 和 Cu 2+
摘要 本研究的目的是制备镁铁氧体(MgFe2O4)磁性纳米颗粒,并研究其对水溶液中Mn2+、Co2+、Ni2+、Cu2+离子的吸附特性。MgFe2O4 是用甘氨酸-硝酸盐燃烧法合成的,其特征是结晶度低,晶粒尺寸为 8.2 nm,颗粒聚集体为 13-25 nm,BET 表面积为 14 m2/g,孔径为 8.0 nm。使用单组分模型溶液研究了 MgFe2O4 对 Mn2+、Co2+、Ni2+、Cu2+ 离子的吸附特性,发现其依赖于金属离子浓度、接触时间、pH 值和再生实验条件。MgFe2O4 对 Co2+ (2.30 mmol g1) 和 Mn2+ (1.56 mmol g-1) 的吸附能力最高,对 Ni2+ (0.89 mmol g-1) 和 Cu2+ (0.46 mmol g-1) 的吸附能力最低。据观察,吸附平衡在 20-60 分钟内很快发生。吸附剂的 pHzpc 计算为 6.58。在研究的 pH 区间 (3.0-7.0) 中,MgFe2O4 的吸附能力没有受到显着影响。再生研究表明,负载金属的吸附剂可以在 120 分钟的接触时间内通过 pH 6.0 的 10-3 M MgCl2 水溶液再生。再生试验表明,MgFe2O4 磁性吸附剂至少可以有效地使用四个吸附-解吸循环。有毒金属离子吸附的高吸附特性和动力学表明开发的吸附剂在废水处理实践中具有良好的前景。0) MgFe2O4 的吸附能力没有受到显着影响。再生研究表明,负载金属的吸附剂可以在 120 分钟的接触时间内通过 pH 6.0 的 10-3 M MgCl2 水溶液再生。再生试验表明,MgFe2O4 磁性吸附剂至少可以有效地使用四个吸附-解吸循环。有毒金属离子吸附的高吸附特性和动力学表明开发的吸附剂在废水处理实践中具有良好的前景。0) MgFe2O4 的吸附能力没有受到显着影响。再生研究表明,负载金属的吸附剂可以在 120 分钟的接触时间内通过 pH 6.0 的 10-3 M MgCl2 水溶液再生。再生试验表明,MgFe2O4 磁性吸附剂至少可以有效地使用四个吸附-解吸循环。有毒金属离子吸附的高吸附特性和动力学表明开发的吸附剂在废水处理实践中具有良好的前景。
更新日期:2018-06-01
中文翻译:

铁氧体镁纳米颗粒作为磁性吸附剂,用于从水溶液中去除 Mn 2+ 、Co 2+ 、Ni 2+ 和 Cu 2+
摘要 本研究的目的是制备镁铁氧体(MgFe2O4)磁性纳米颗粒,并研究其对水溶液中Mn2+、Co2+、Ni2+、Cu2+离子的吸附特性。MgFe2O4 是用甘氨酸-硝酸盐燃烧法合成的,其特征是结晶度低,晶粒尺寸为 8.2 nm,颗粒聚集体为 13-25 nm,BET 表面积为 14 m2/g,孔径为 8.0 nm。使用单组分模型溶液研究了 MgFe2O4 对 Mn2+、Co2+、Ni2+、Cu2+ 离子的吸附特性,发现其依赖于金属离子浓度、接触时间、pH 值和再生实验条件。MgFe2O4 对 Co2+ (2.30 mmol g1) 和 Mn2+ (1.56 mmol g-1) 的吸附能力最高,对 Ni2+ (0.89 mmol g-1) 和 Cu2+ (0.46 mmol g-1) 的吸附能力最低。据观察,吸附平衡在 20-60 分钟内很快发生。吸附剂的 pHzpc 计算为 6.58。在研究的 pH 区间 (3.0-7.0) 中,MgFe2O4 的吸附能力没有受到显着影响。再生研究表明,负载金属的吸附剂可以在 120 分钟的接触时间内通过 pH 6.0 的 10-3 M MgCl2 水溶液再生。再生试验表明,MgFe2O4 磁性吸附剂至少可以有效地使用四个吸附-解吸循环。有毒金属离子吸附的高吸附特性和动力学表明开发的吸附剂在废水处理实践中具有良好的前景。0) MgFe2O4 的吸附能力没有受到显着影响。再生研究表明,负载金属的吸附剂可以在 120 分钟的接触时间内通过 pH 6.0 的 10-3 M MgCl2 水溶液再生。再生试验表明,MgFe2O4 磁性吸附剂至少可以有效地使用四个吸附-解吸循环。有毒金属离子吸附的高吸附特性和动力学表明开发的吸附剂在废水处理实践中具有良好的前景。0) MgFe2O4 的吸附能力没有受到显着影响。再生研究表明,负载金属的吸附剂可以在 120 分钟的接触时间内通过 pH 6.0 的 10-3 M MgCl2 水溶液再生。再生试验表明,MgFe2O4 磁性吸附剂至少可以有效地使用四个吸附-解吸循环。有毒金属离子吸附的高吸附特性和动力学表明开发的吸附剂在废水处理实践中具有良好的前景。



























 京公网安备 11010802027423号
京公网安备 11010802027423号