当前位置:
X-MOL 学术
›
Anal. Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigation of ethosuximide stability under certain ICH-recommended stress conditions using a validated stability-indicating HPLC method
Analytical Methods ( IF 2.7 ) Pub Date : 2018-02-13 00:00:00 , DOI: 10.1039/c7ay02740k Y. El-Shabrawy 1, 2, 3, 4, 5 , M. Walash 1, 2, 3, 4, 5 , N. El-Enany 1, 2, 3, 4, 5 , R. El-Shaheny 1, 2, 3, 4, 5
Analytical Methods ( IF 2.7 ) Pub Date : 2018-02-13 00:00:00 , DOI: 10.1039/c7ay02740k Y. El-Shabrawy 1, 2, 3, 4, 5 , M. Walash 1, 2, 3, 4, 5 , N. El-Enany 1, 2, 3, 4, 5 , R. El-Shaheny 1, 2, 3, 4, 5
Affiliation
|
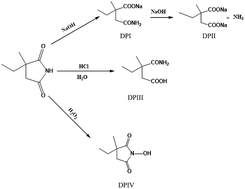
A study of the chemical stability of the antiepileptic drug ethosuximide (ESX) under certain ICH-recommended stress conditions was conducted for the first time. A stability-indicating HPLC method was applied using a mobile phase containing 0.05 M sodium dihydrogen phosphate and methanol (90 : 10 v/v) at pH 3.5 and a Promosil C18 column for separation of ESX from its potential degradation products with UV detection at 210 nm. The method validity was confirmed showing a linearity range of 2.0–30.0 μg mL−1 with a lower limit of detection of 0.14 μg mL−1. The results of the developed method showed good agreement with those obtained by the USP official method. The stability of ESX was investigated by subjecting it to some stress conditions of the ICH guideline such as acidic, alkaline and oxidative conditions. The drug was liable to degradation under these conditions yielding specific degradation products. The validated stability-indicating HPLC method efficiently separated the drug from all of the formed degradation products proving its suitability for purity and stability testing.
中文翻译:

使用经过验证的稳定性指示HPLC方法研究在某些ICH推荐的应力条件下乙琥胺的稳定性
首次进行了在某些ICH推荐的应激条件下抗癫痫药ethosuximide(ESX)的化学稳定性的研究。使用指示稳定性的HPLC方法,使用的流动相包含pH 3.5的0.05 M磷酸二氢钠和甲醇(90:10 v / v)和Promosil C 18色谱柱,用于在潜在的降解产物中分离ESX,并通过UV检测。 210纳米 确认方法的有效性,显示线性范围为2.0–30.0μgmL -1,检测下限为0.14μgmL -1。所开发方法的结果与通过USP官方方法获得的结果吻合良好。通过使ESX经受ICH准则的某些应力条件(例如酸性,碱性和氧化条件),可以研究其稳定性。该药物在这些条件下易于降解,产生特定的降解产物。经过验证的稳定性指示HPLC方法可有效地从所有形成的降解产物中分离药物,证明其适用于纯度和稳定性测试。
更新日期:2018-02-13
中文翻译:

使用经过验证的稳定性指示HPLC方法研究在某些ICH推荐的应力条件下乙琥胺的稳定性
首次进行了在某些ICH推荐的应激条件下抗癫痫药ethosuximide(ESX)的化学稳定性的研究。使用指示稳定性的HPLC方法,使用的流动相包含pH 3.5的0.05 M磷酸二氢钠和甲醇(90:10 v / v)和Promosil C 18色谱柱,用于在潜在的降解产物中分离ESX,并通过UV检测。 210纳米 确认方法的有效性,显示线性范围为2.0–30.0μgmL -1,检测下限为0.14μgmL -1。所开发方法的结果与通过USP官方方法获得的结果吻合良好。通过使ESX经受ICH准则的某些应力条件(例如酸性,碱性和氧化条件),可以研究其稳定性。该药物在这些条件下易于降解,产生特定的降解产物。经过验证的稳定性指示HPLC方法可有效地从所有形成的降解产物中分离药物,证明其适用于纯度和稳定性测试。











































 京公网安备 11010802027423号
京公网安备 11010802027423号