当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An atmosphere and light tuned highly diastereoselective synthesis of cyclobuta/penta[b]indoles from aniline-tethered alkylidenecyclopropanes with alkynes
Chemical Communications ( IF 4.3 ) Pub Date : 2018-02-13 00:00:00 , DOI: 10.1039/c8cc00180d Bo Cao 1, 2, 3, 4, 5 , Yin Wei 6, 7, 8, 9, 10 , Min Shi 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2018-02-13 00:00:00 , DOI: 10.1039/c8cc00180d Bo Cao 1, 2, 3, 4, 5 , Yin Wei 6, 7, 8, 9, 10 , Min Shi 1, 2, 3, 4, 5
Affiliation
|
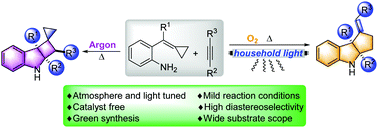
Metal-free and environmentally friendly synthesis highly controlled by natural sources like visible light and air (or oxygen) is always a pursuit of green chemistry. In this paper, an atmosphere and light tuned highly diastereoselective synthesis of two important polyheterocyclic skeletons: cyclobuta/penta[b]indoles from aniline-tethered alkylidenecyclopropanes with alkynes, has been developed. The chiral cyclobuta/penta[b]indoles have also been obtained by optical resolution.
中文翻译:

由苯胺系链的亚烷基环丙烷与炔烃在气氛和光调谐下高度非对映选择性合成环丁/戊[ b ]吲哚
受到可见光和空气(或氧气)等自然资源高度控制的无金属,环保的合成方法一直是绿色化学的追求。在本文中,气氛和光调谐两个重要的多杂环骨架高度非对映合成:cyclobuta /五[ b ]从与炔苯胺拴alkylidenecyclopropanes吲哚,已经研制成功。还通过光学拆分获得了手性环丁/戊[ b ]吲哚。
更新日期:2018-03-15
中文翻译:

由苯胺系链的亚烷基环丙烷与炔烃在气氛和光调谐下高度非对映选择性合成环丁/戊[ b ]吲哚
受到可见光和空气(或氧气)等自然资源高度控制的无金属,环保的合成方法一直是绿色化学的追求。在本文中,气氛和光调谐两个重要的多杂环骨架高度非对映合成:cyclobuta /五[ b ]从与炔苯胺拴alkylidenecyclopropanes吲哚,已经研制成功。还通过光学拆分获得了手性环丁/戊[ b ]吲哚。











































 京公网安备 11010802027423号
京公网安备 11010802027423号