当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel valdecoxib derivatives by ruthenium(ii)-promoted 1,3-dipolar cycloaddition of nitrile oxides with alkynes – synthesis and COX-2 inhibition activity†
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2018-02-13 00:00:00 , DOI: 10.1039/c7md00575j Silvia Roscales 1, 2, 3, 4, 5 , Nicole Bechmann 1, 2, 3, 4, 5 , Daniel Holger Weiss 5, 6, 7, 8, 9 , Martin Köckerling 5, 6, 7, 8, 9 , Jens Pietzsch 1, 2, 3, 4, 5 , Torsten Kniess 1, 2, 3, 4, 5
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2018-02-13 00:00:00 , DOI: 10.1039/c7md00575j Silvia Roscales 1, 2, 3, 4, 5 , Nicole Bechmann 1, 2, 3, 4, 5 , Daniel Holger Weiss 5, 6, 7, 8, 9 , Martin Köckerling 5, 6, 7, 8, 9 , Jens Pietzsch 1, 2, 3, 4, 5 , Torsten Kniess 1, 2, 3, 4, 5
Affiliation
|
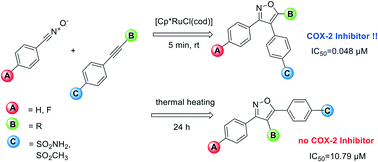
Novel valdecoxib-based cyclooxygenase-2 inhibitors were synthesized in one step via 1,3-dipolar cycloaddition of nitrile oxides with a series of eleven aryl alkynes, six of them described for the first time. Application of Ru(II)-catalysis leads preferably to the formation of the 3,4-diaryl-substituted isoxazoles, while under thermal heating with base the 3,5-diaryl substitution pattern is favoured. The new the 3,4-diaryl-substituted isoxazoles possessing a small substituent (H and Me) displayed high COX-2 inhibition affinity (IC50 = 0.042–0.073 μM) and excellent selectivity (COX-2 SI > 2000). In contrast, the 3,5-diaryl substituted compounds displayed almost no COX activity. The introduction of a 4-fluorophenyl substituent resulted in retained high COX-2 affinity, making these compounds together with the feasible one step reaction promising candidates for the development of fluorine-18 labelled radiotracers.
中文翻译:

钌(ii)促进的带有炔烃的腈类氧化物的新型瓦尔德考昔衍生物的合成(1,3-偶极环加成反应)–合成和COX-2抑制活性†
通过将腈与一系列十一个芳基炔烃进行1,3-偶极环加成反应,一步合成了一种新的基于valdecoxib的环氧合酶-2抑制剂,其中六个首次被描述。Ru(II)催化的应用优选导致3,4-二芳基取代的异恶唑的形成,而在与碱一起热加热下,有利于3,5-二芳基取代的模式。具有小的取代基(H和Me)的新的3,4-二芳基取代的异恶唑显示出高的COX-2抑制亲和力(IC 50= 0.042–0.073μM)和优异的选择性(COX-2 SI> 2000)。相反,3,5-二芳基取代的化合物几乎没有表现出COX活性。4-氟苯基取代基的引入导致保留了较高的COX-2亲和力,使这些化合物与可行的一步反应一起有望成为开发氟18标记的放射性示踪剂的候选物。
更新日期:2018-02-13
中文翻译:

钌(ii)促进的带有炔烃的腈类氧化物的新型瓦尔德考昔衍生物的合成(1,3-偶极环加成反应)–合成和COX-2抑制活性†
通过将腈与一系列十一个芳基炔烃进行1,3-偶极环加成反应,一步合成了一种新的基于valdecoxib的环氧合酶-2抑制剂,其中六个首次被描述。Ru(II)催化的应用优选导致3,4-二芳基取代的异恶唑的形成,而在与碱一起热加热下,有利于3,5-二芳基取代的模式。具有小的取代基(H和Me)的新的3,4-二芳基取代的异恶唑显示出高的COX-2抑制亲和力(IC 50= 0.042–0.073μM)和优异的选择性(COX-2 SI> 2000)。相反,3,5-二芳基取代的化合物几乎没有表现出COX活性。4-氟苯基取代基的引入导致保留了较高的COX-2亲和力,使这些化合物与可行的一步反应一起有望成为开发氟18标记的放射性示踪剂的候选物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号