当前位置:
X-MOL 学术
›
J. Ind. Eng. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Obtaining glycerol carbonate and glycols using thermomorphic systems based on glycerol and cyclic organic carbonates: kinetic studies
Journal of Industrial and Engineering Chemistry ( IF 5.9 ) Pub Date : 2018-07-01 , DOI: 10.1016/j.jiec.2018.02.008 Jesus Esteban , Andreas J. Vorholt
Journal of Industrial and Engineering Chemistry ( IF 5.9 ) Pub Date : 2018-07-01 , DOI: 10.1016/j.jiec.2018.02.008 Jesus Esteban , Andreas J. Vorholt
|
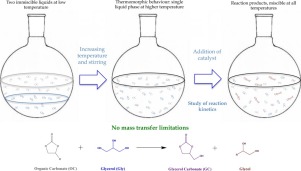
Abstract Glycerol carbonate has mostly been synthesized by transesterification of glycerol with dimethyl carbonate and ethylene carbonate. We propose to obtain it using propylene and butylene carbonate as co-substrates with Na2CO3 as catalyst, also generating 1,2-propanediol and 1,2-butanediol. The reactants exhibit thermomorphic behavior, so we capitalized on this feature to avoid mass transfer limitations instead of using co-solvents. The kinetics of both reactions were studied, obtaining activation energies and deactivation constants of 67.13 ± 1.82 kJ mol−1 and 0.14 ± 0.02 min−1 for the reaction between glycerol and propylene carbonate and 58.56 ± 1.10 kJ mol−1 and 0.39 ± 0.03 min−1 for the reaction of glycerol with butylene carbonate.
中文翻译:

使用基于甘油和环状有机碳酸酯的热形系统获得甘油碳酸酯和二醇:动力学研究
摘要 碳酸甘油酯主要通过甘油与碳酸二甲酯和碳酸亚乙酯的酯交换反应合成。我们建议使用碳酸亚丙酯和碳酸亚丁酯作为共底物,以 Na2CO3 作为催化剂获得它,同时生成 1,2-丙二醇和 1,2-丁二醇。反应物表现出热形行为,因此我们利用这一特征来避免传质限制,而不是使用共溶剂。研究了两种反应的动力学,获得了 67.13 ± 1.82 kJ mol-1 和 0.14 ± 0.02 min-1 的活化能和失活常数,用于甘油和碳酸亚丙酯之间的反应以及 58.56 ± 1.10 kJ mol-1 和 0.39 ± 0.03 min -1 用于甘油与碳酸亚丁酯的反应。
更新日期:2018-07-01
中文翻译:

使用基于甘油和环状有机碳酸酯的热形系统获得甘油碳酸酯和二醇:动力学研究
摘要 碳酸甘油酯主要通过甘油与碳酸二甲酯和碳酸亚乙酯的酯交换反应合成。我们建议使用碳酸亚丙酯和碳酸亚丁酯作为共底物,以 Na2CO3 作为催化剂获得它,同时生成 1,2-丙二醇和 1,2-丁二醇。反应物表现出热形行为,因此我们利用这一特征来避免传质限制,而不是使用共溶剂。研究了两种反应的动力学,获得了 67.13 ± 1.82 kJ mol-1 和 0.14 ± 0.02 min-1 的活化能和失活常数,用于甘油和碳酸亚丙酯之间的反应以及 58.56 ± 1.10 kJ mol-1 和 0.39 ± 0.03 min -1 用于甘油与碳酸亚丁酯的反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号