Chemical Physics ( IF 2.0 ) Pub Date : 2018-02-12 , DOI: 10.1016/j.chemphys.2018.02.013 Jingni Fu , Luning Zhang
|
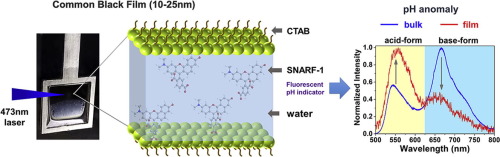
The protonation/deprotonation equilibrium of a fluorescent pH probe (carboxy-seminaphthorhodafluor-1, SNARF-1) within the nanoscale water layer confined in common black films (CBFs) has been studied. We find that SNARF-1 molecules feel a more acidic environment in CBFs than when they are in the bulk micellar solution, using the base/acid peak area ratio of the dye to indicate its microenvironment pH. Three surfactants are used to study the dependence of the pH drop versus charge: cationic (cetyltrimethylammonium bromide, CTAB), anionic (sodium dodecylsulphate, SDS) and nonionic (Triton X-100) species. The decrease of CBFs pH versus the pH of the micellar solution is the following: ΔpH≈1.5 for CTAB (pH: 7.0-9.0), ΔpH≈0.8 for SDS, and ΔpH≈0.4 for Triton X-100. With the addition of electrolyte in CBFs, we observe large decrease the amplitude of the pH anomaly, thus suggesting an electrostatic origin of the pH change at nanoscale environment.
中文翻译:

用荧光染料探究普通黑色薄膜中胶束溶液与纳米级水之间的pH差异
研究了局限在普通黑膜(CBF)中的纳米级水层中的荧光pH探针(羧基-seminaphthorhodafluor-1,SNARF-1)的质子/去质子平衡。我们发现,SNARF-1分子在CBF中的酸性环境比在散装胶束溶液中的酸性环境大,使用染料的碱/酸峰面积比来表明其微环境pH。三种表面活性剂用于研究pH下降对电荷的依赖性:阳离子型(十六烷基三甲基溴化铵,CTAB),阴离子型(十二烷基硫酸钠,SDS)和非离子型(Triton X-100)。CBFs pH相对于胶束溶液pH的降低如下:CTAB的ΔpH≈1.5(pH:7.0-9.0),SDS的ΔpH≈0.8,Triton X-100的ΔpH≈0.4。在CBF中添加电解质后,









































 京公网安备 11010802027423号
京公网安备 11010802027423号