当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Boosting the Potassium Storage Performance of Alloy‐Based Anode Materials via Electrolyte Salt Chemistry
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2018-02-12 , DOI: 10.1002/aenm.201703288 Qing Zhang 1 , Jianfeng Mao 1 , Wei Kong Pang 1 , Tian Zheng 2 , Vitor Sencadas 3 , Yuanzhen Chen 1 , Yajie Liu 1 , Zaiping Guo 1, 3
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2018-02-12 , DOI: 10.1002/aenm.201703288 Qing Zhang 1 , Jianfeng Mao 1 , Wei Kong Pang 1 , Tian Zheng 2 , Vitor Sencadas 3 , Yuanzhen Chen 1 , Yajie Liu 1 , Zaiping Guo 1, 3
Affiliation
|
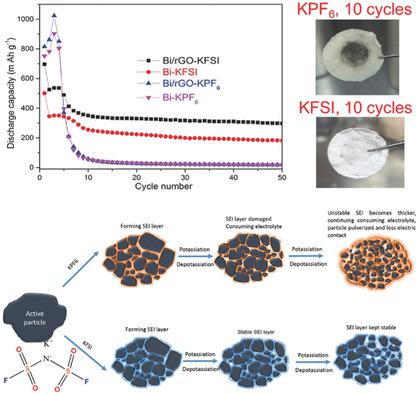
Potassium‐ion batteries (PIBs) are promising energy storage systems because of the abundance and low cost of potassium. The formidable challenge is to develop suitable electrode materials and electrolytes for accommodating the relatively large size and high activity of potassium. Herein, Bi‐based materials are reported as novel anodes for PIBs. Nanostructural design and proper selection of the electrolyte salt have been used to achieve excellent cycling performance. It is found that the potassiation of Bi undergoes a solid‐solution reaction, followed by two typical two‐phase reactions, corresponding to Bi ↔ Bi(K) and Bi(K) ↔ K5Bi4 ↔ K3Bi, respectively. By choosing potassium bis(fluorosulfonyl)imide (KFSI) to replace potassium hexafluorophosphate (KPF6) in carbonate electrolyte, a more stable solid electrolyte interphase layer is achieved and results in notably enhanced electrochemical performance. More importantly, the KFSI salt is very versatile and can significantly promote the electrochemical performance of other alloy‐based anode materials, such as Sn and Sb.
中文翻译:

通过电解质盐化学提高合金基负极材料的钾储存性能
钾离子电池(PIB)由于钾的丰富和低成本而成为有前途的储能系统。艰巨的挑战是开发合适的电极材料和电解质,以适应钾的较大尺寸和高活性。在本文中,Bi-基材料据报道是PIB的新型阳极。纳米结构设计和电解质盐的正确选择已用于实现出色的循环性能。据发现,Bi的potassiation经历固溶反应,接着进行两个典型的两相反应中,对应于毕毕↔(K)和Bi(K)↔ķ 5的Bi 4 ↔ķ 3的Bi,分别。通过选择双(氟磺酰基)酰亚胺钾(KFSI)替代六氟磷酸钾(KPF6)在碳酸盐电解质中,获得了更稳定的固体电解质界面层,并导致电化学性能显着增强。更重要的是,KFSI盐用途广泛,可以显着提高其他基于合金的负极材料(例如Sn和Sb)的电化学性能。
更新日期:2018-02-12
中文翻译:

通过电解质盐化学提高合金基负极材料的钾储存性能
钾离子电池(PIB)由于钾的丰富和低成本而成为有前途的储能系统。艰巨的挑战是开发合适的电极材料和电解质,以适应钾的较大尺寸和高活性。在本文中,Bi-基材料据报道是PIB的新型阳极。纳米结构设计和电解质盐的正确选择已用于实现出色的循环性能。据发现,Bi的potassiation经历固溶反应,接着进行两个典型的两相反应中,对应于毕毕↔(K)和Bi(K)↔ķ 5的Bi 4 ↔ķ 3的Bi,分别。通过选择双(氟磺酰基)酰亚胺钾(KFSI)替代六氟磷酸钾(KPF6)在碳酸盐电解质中,获得了更稳定的固体电解质界面层,并导致电化学性能显着增强。更重要的是,KFSI盐用途广泛,可以显着提高其他基于合金的负极材料(例如Sn和Sb)的电化学性能。







































 京公网安备 11010802027423号
京公网安备 11010802027423号