当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Novel 3‐substituted N‐methylcarbazole–imidazolium salt derivatives: Synthesis and cytotoxic activity
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2018-03-03 , DOI: 10.1111/cbdd.13178 Yan-Hua Li 1 , Bei Zhou 2 , Yi-Min Shi 1 , Yu-Peng Xun 1 , Yun-Han Yang 1 , Li-Juan Yang 1
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2018-03-03 , DOI: 10.1111/cbdd.13178 Yan-Hua Li 1 , Bei Zhou 2 , Yi-Min Shi 1 , Yu-Peng Xun 1 , Yun-Han Yang 1 , Li-Juan Yang 1
Affiliation
|
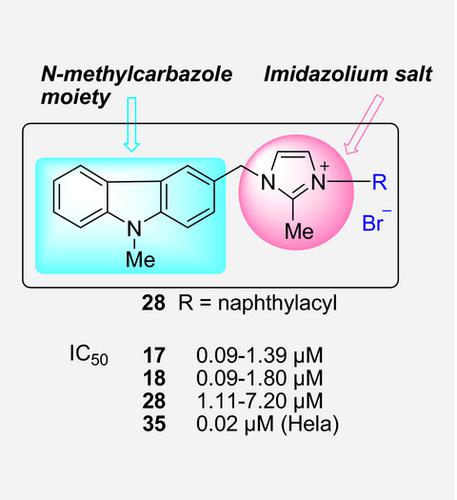
A series of novel 3‐substituted N‐methylcarbazole–imidazolium salt derivatives has been prepared and evaluated in vitro against a panel of tumor cell lines (Hep G‐2, Hela and PC12). The results suggest that the presence of substituted 2‐methyl‐imidazole or imidazole ring and substitution of the imidazolyl‐3‐position with a naphthylacyl or 4‐bromophenacyl group were important for improving cytotoxic activity. Compounds 17, 18, 27, and 28 with 4‐bromophenacyl and naphthylacyl groups displayed good activities with IC50 values of 0.09–7.20 μm against three tumor cell lines investigated and more active than DDP. Compound 35 exhibited cytotoxic activity selectively against Hela cell.
中文翻译:

新型3-取代的N-甲基咔唑-咪唑鎓盐衍生物:合成和细胞毒性活性
已经制备了一系列新颖的3-取代的N-甲基咔唑-咪唑鎓盐衍生物,并针对一组肿瘤细胞系(Hep G-2,Hela和PC12)进行了体外评估。结果表明,取代的2-甲基咪唑或咪唑环的存在以及咪唑基-3-位置被萘甲酰基或4-溴苯甲酰基取代对于提高细胞毒性活性很重要。化合物17,18,27,和28与4-溴苯甲酰和naphthylacyl基团表现出良好的活性与IC 50个的0.09-7.20μ值米针对三种肿瘤细胞系研究和比DDP更活跃。化合物35 表现出针对Hela细胞的选择性细胞毒活性。
更新日期:2018-03-03
中文翻译:

新型3-取代的N-甲基咔唑-咪唑鎓盐衍生物:合成和细胞毒性活性
已经制备了一系列新颖的3-取代的N-甲基咔唑-咪唑鎓盐衍生物,并针对一组肿瘤细胞系(Hep G-2,Hela和PC12)进行了体外评估。结果表明,取代的2-甲基咪唑或咪唑环的存在以及咪唑基-3-位置被萘甲酰基或4-溴苯甲酰基取代对于提高细胞毒性活性很重要。化合物17,18,27,和28与4-溴苯甲酰和naphthylacyl基团表现出良好的活性与IC 50个的0.09-7.20μ值米针对三种肿瘤细胞系研究和比DDP更活跃。化合物35 表现出针对Hela细胞的选择性细胞毒活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号