Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2018-02-10 , DOI: 10.1016/j.jfluchem.2018.02.007 Charlotte S. Teschers , Constantin G. Daniliuc , Gerald Kehr , Ryan Gilmour
|
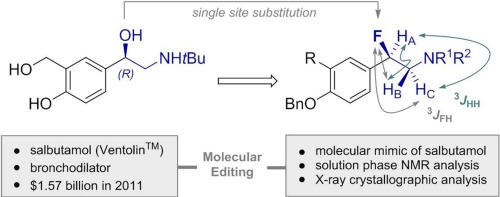
The bronchodilator salbutamol adopts a characteristic gauche conformation about the ϕO-C-C-N torsion angle. This topology is predicated on stabilizing stereoelectronic interactions of the type σ → σC-X*. X-ray crystallographic analysis of salbutamol also indicates that an intramolecular hydrogen bond reinforces this intuitive conformation (ϕO-C-C-N ≈ 60°). In this study, we demonstrate that single site OH → F substitution in model salbutamol systems preserves the gauche conformational preference by virtue of reinforcing hyperconjugative interactions of the type σC-H → σC-F* and σC-C → σC-N*. Since the amine remains fully protected throughout this conformational analysis, intramolecular hydrogen bonding can be discounted. Conformational mimesis is confirmed by NMR spectroscopy in solution, and also in the solid state.
中文翻译:

构象控制启用由氟笨拙在β的模型效果2 -AR激动剂沙丁胺醇(托林™)
支气管扩张药沙丁胺醇在ϕ O-CCN扭转角附近采用特征性的gauche构型。该拓扑基于稳定的σ→σC -X *类型的立体电子相互作用。沙丁胺醇的X射线晶体学分析还表明,分子内氢键增强了这种直观构象(ϕ O-CCN≈60 °)。在这项研究中,我们证明了模型沙丁胺醇系统中的单位OH→F取代通过增强σC -H →σC -F *和σC -C →σC -N类型的超共轭相互作用而保留了gauche构象偏好。*。由于胺在整个构象分析过程中保持完全被保护,因此分子内氢键可被打折。通过NMR光谱在溶液中以及在固态下也证实了构象模仿。











































 京公网安备 11010802027423号
京公网安备 11010802027423号