Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2018-02-10 , DOI: 10.1016/j.jfluchem.2018.02.006 Afsaneh Asr , Saeedreza Emamian , Mehran Aghaie , Hossein Aghaie
|
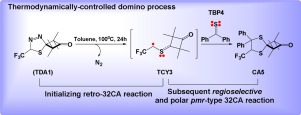
A CF3-containing thiadiazole derivative, TDA1, participates in a domino process initialized with a thermal N2 extrusion experienced at TDA1 within a retro- [3+2] cycloaddition (32CA) reaction to yield CF3-substituted thiocarbonyl ylide TCY3. Then, TYC3 is trapped over the course of a subsequent 32CA reaction toward thioketone TBP4 to generate dithiolane derivative CA5. This domino process was studied through Molecular Electron Density Theory (MEDT) at the B3LYP/6-31G(d) computational level. Exploration of the relative Gibbs free energies obviously indicates that this domino process needs to overcome high barriers justifying why the harsh conditions (100 °C for 24h in toluene) is experimentally requested. In excellent agreement with the experimental findings, an entirely C1–C5 regioselective, polar, and pmr-type 32CA reaction of in situ generated TCY3 toward TBP4 leads to the formation of cycloadduct CA5 as the sole product along a quite irreversible pathway. While the high global electrophilicity ω index of TBP4 together with the high global nucleophilicity N index of TCY3 are responsible for the considerable polar character of the mentioned 32CA reaction, analysis of the electrophilic and nucleophilic Parr functions at the reactive sites of reactants allows to rationalize C1–C5 regioselectivity observed experimentally. In terms of quantum topological analysis of the electron localization function (ELF) over some relevant points located along the IRC profile connecting separate TCY3 and TBP4 to CA5, a non-concerted two-stage one-step molecular mechanism is established for this polar pmr-type 32CA reaction. Such ELF analysis evidently shows that formation of the first C1–C5 and second C3–S4 single bonds is a direct consequence of coupling C1- to –C5 and C3- to –S4 pseudoradical centers, respectively.
中文翻译:

CF 3取代的硫代羰基烷基化物与硫代酮之间的[3 + 2]环加成反应:使用分子电子密度理论探索区域选择性和机理方面
含CF 3的噻二唑衍生物TDA1参与多米诺骨牌过程,该过程由在逆向[3 + 2]环加成(32CA)反应中在TDA1经历的热N 2挤出而初始化,从而生成CF 3取代的硫代羰基内酯TCY3。然后,TYC3被截留在后续32CA反应过程朝向硫酮TBP4以产生二硫戊环衍生物CA5。通过分子电子密度理论(MEDT)在B3LYP / 6-31G(d)的计算水平上研究了该多米诺过程。对相对吉布斯自由能的探索显然表明,这种多米诺骨牌工艺需要克服高障碍,从而证明了为什么需要实验性地要求苛刻的条件(在甲苯中100°C放置24小时)。与实验结果非常吻合,原位生成的TCY3向TBP4的完全C1-C5区域选择性,极性和pmr型32CA反应导致环加合物CA5沿唯一不可逆的途径形成。而TBP4的高整体亲电性ω指数TCY3的高全球亲核性N指数与上述32CA反应的相当大的极性有关,对亲电子性进行了分析 和亲核的 在反应物的反应位点的Parr功能可以使实验观察到的C1-C5区域选择性合理化。根据沿IRC轮廓将独立的TCY3和TBP4连接到CA5的IRC轮廓上的一些相关点的电子局部化功能(ELF)的量子拓扑分析,为此极性pmr-建立了非证明性的两阶段单步分子机制。32CA型反应。这种ELF分析显然表明,第一个C1-C5和第二个C3-S4单键的形成是分别将C1-到–C5和C3到–S4伪自由基中心耦合的直接结果。










































 京公网安备 11010802027423号
京公网安备 11010802027423号