Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2018-02-10 , DOI: 10.1016/j.jinorgbio.2018.02.007 Birgitte Jensen , Angela Fago
|
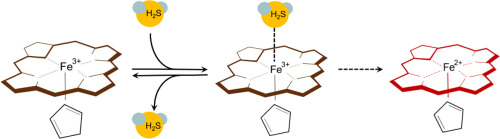
Ferric hemoglobin (metHb) and myoglobin (metMb), present at low levels in vivo, have been recently found to oxidize hydrogen sulfide (H2S) in excess, thus potentially contributing to removal of toxic H2S in blood and heart, respectively. Here, we present a kinetic and thermodynamic study of the reaction of metHb and metMb with H2S under physiological conditions, i.e. at low H2S concentrations and with protein in excess of H2S. We show here that both proteins react with sub-stoichiometric H2S:heme ratios following two processes: a fast reversible binding of H2S to ferric heme that prevails at high H2S and a slow heme reduction to the ferrous state that prevails at low H2S. While these two processes are fast for metMb, H2S-induced heme reduction is slow for metHb and the metHb-H2S complex once formed is therefore relatively stable. We find that metHb binds H2S reversibly and cooperatively with a pH-dependent ligand affinity that is within the physiological range of H2S concentrations found in blood. Stopped-flow kinetics show identical association rate constants for H2S at varying pH, demonstrating that H2S and not HS− enters the ferric heme pocket. Dissociation rates of the metHb-H2S complex increase when decreasing pH, consistent with the pH-dependent affinity. Taken together, these data are consistent with a novel biological role of metHb as a H2S carrier in the blood, in parallel with the oxygen carrier function of the much more abundant ferrous Hb. In contrast, metMb in the heart could participate to redox-signaling involving H2S.
中文翻译:

生理条件下三价铁血红蛋白和肌红蛋白与硫化氢的反应
最近发现体内存在的三价铁血红蛋白(metHb)和肌红蛋白(metMb)会过量氧化硫化氢(H 2 S),因此分别潜在地有助于去除血液和心脏中的有毒H 2 S 。这里,我们提出metHb和metMb的用H的反应的动力学和热力学研究2的生理条件下,即在低ħ 2硫的浓度,并用过量H的蛋白2 S.我们在这里表明,这两种蛋白质与子反应化学计量比的H 2 S:血红素比率可通过以下两个过程进行:H 2 S与三价铁血红素的快速可逆结合,在高H时占优势2 S和在低H 2 S时普遍存在的亚铁血红素缓慢还原。虽然这两个过程对于metMb快速,但是对于metHb H 2 S诱导的血红素还原缓慢,并且一旦形成了metHb-H 2 S复合物。因此相对稳定。我们发现metHb可逆地结合H 2 S并与pH依赖性配体亲和力结合,该亲和力在血液中发现的H 2 S浓度的生理范围内。停流动力学显示用于h相同结合速率常数2 S中变化的pH,表明ħ 2 S和不HS -进入铁血红素口袋。metHb-H 2的解离速率当降低pH时,S络合物增加,这与pH依赖性亲和力一致。综上所述,这些数据与作为血液中H 2 S载体的metHb的新型生物学作用,以及更为丰富的亚铁Hb的氧载体功能相一致。相反,心脏中的metMb可能参与涉及H 2 S的氧化还原信号传递。











































 京公网安备 11010802027423号
京公网安备 11010802027423号