Electrochemistry Communications ( IF 4.7 ) Pub Date : 2018-02-09 , DOI: 10.1016/j.elecom.2018.02.006 Kazuhiko Mukai , Takao Inoue
|
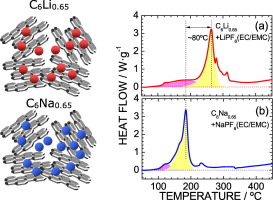
The thermal stabilities of Na- and Li-intercalated hard carbons were examined by differential scanning calorimetry (DSC) to determine whether Na-ion batteries or Li-ion batteries exhibit superior thermal stability. The DSC profiles of both C6Na0.65 and C6Li0.65 displayed sharp exothermic peaks with enthalpy changes of ~1200 J·g−1, which could be attributed to the reaction between C6Na0.65 (or C6Li0.65) and PF5. Hence, the total amounts of heat generated in these Na- and Li-ion batteries were equal, regardless of the intercalants present. However, the temperatures corresponding to the maximum exothermic peaks differed between the two battery systems (i.e., 184 °C for C6Na0.65 and 264 °C for C6Li0.65), indicating that Na-ion batteries are less thermally stable. The DSC profile of C6Li0.25Na0.37 revealed that this difference is due to differences in the solid-electrolyte interphase films between C6Nax and C6Liy.
中文翻译:

通过差示扫描量热法区分Na和Li插入的硬碳的热行为
通过差示扫描量热法(DSC)检查了插有Na和Li的硬碳的热稳定性,以确定Na离子电池或Li离子电池是否表现出优异的热稳定性。C 6 Na 0.65和C 6 Li 0.65的DSC谱均显示出尖锐的放热峰,焓变约为1200 J·g -1,这可能归因于C 6 Na 0.65(或C 6 Li 0.65)与C 6 Na 0.65之间的反应。PF 5。因此,无论存在哪种嵌入剂,在这些Na和Li离子电池中产生的热量总量都是相等的。但是,在两个电池系统之间,对应于最大放热峰的温度有所不同(即C 6 Na 0.65为184 °C,C 6 Li 0.65为264°C ),这表明Na离子电池的热稳定性较差。C 6 Li 0.25 Na 0.37的DSC图谱表明,该差异是由于C 6 Na x和C 6 Li y之间的固体电解质中间相膜的差异引起的。









































 京公网安备 11010802027423号
京公网安备 11010802027423号