当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tumor Targeting with Novel Pyridyl 6-Substituted Pyrrolo[2,3-d]Pyrimidine Antifolates via Cellular Uptake by Folate Receptor α and the Proton-Coupled Folate Transporter and Inhibition of De Novo Purine Nucleotide Biosynthesis
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-02-09 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01708 Manasa Ravindra 1 , Adrianne Wallace-Povirk 2 , Mohammad A. Karim 1 , Mike R. Wilson 2 , Carrie O’Connor 2 , Kathryn White 2 , Juiwanna Kushner 2 , Lisa Polin 2, 3 , Christina George 2 , Zhanjun Hou 2, 3 , Larry H. Matherly 2, 3, 4 , Aleem Gangjee 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-02-09 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01708 Manasa Ravindra 1 , Adrianne Wallace-Povirk 2 , Mohammad A. Karim 1 , Mike R. Wilson 2 , Carrie O’Connor 2 , Kathryn White 2 , Juiwanna Kushner 2 , Lisa Polin 2, 3 , Christina George 2 , Zhanjun Hou 2, 3 , Larry H. Matherly 2, 3, 4 , Aleem Gangjee 1
Affiliation
|
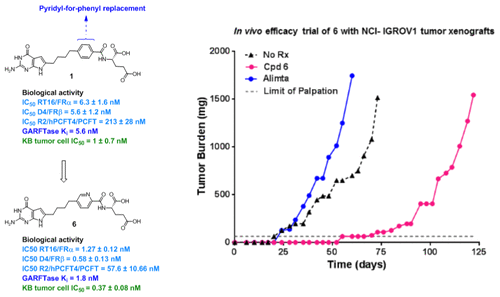
Tumor-targeted specificities of 6-substituted pyrrolo[2,3-d]pyrimidine analogues of 1, where the phenyl side-chain is replaced by 3′,6′ (5, 8), 2′,5′ (6, 9), and 2′,6′ (7, 10) pyridyls, were analyzed. Proliferation inhibition of isogenic Chinese hamster ovary (CHO) cells expressing folate receptors (FRs) α and β were in rank order, 6 > 9 > 5 > 7 > 8, with 10 showing no activity, and 6 > 9 > 5 > 8, with 10 and 7 being inactive, respectively. Antiproliferative effects toward FRα- and FRβ-expressing cells were reflected in competitive binding with [3H]folic acid. Only compound 6 was active against proton-coupled folate receptor (PCFT)-expressing CHO cells (∼4-fold more potent than 1) and inhibited [3H]methotrexate uptake by PCFT. In KB and IGROV1 tumor cells, 6 showed <1 nM IC50, ∼2–3-fold more potent than 1. Compound 6 inhibited glycinamide ribonucleotide formyltransferase in de novo purine biosynthesis and showed potent in vivo efficacy toward subcutaneous IGROV1 tumor xenografts in SCID mice.
更新日期:2018-02-09











































 京公网安备 11010802027423号
京公网安备 11010802027423号