Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2018-02-10 , DOI: 10.1016/j.jinorgbio.2018.02.006 Kun Cao , Jing Zhang , Xin-Yu Miao , Qiu-Xia Wei , Xin-Lu Zhao , Qing-Yu He , Xuesong Sun
|
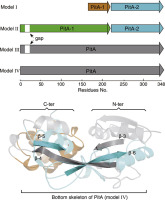
Iron is an essential element for almost all bacteria. The iron ATP-binding cassette (ABC) transporters located on the cell membrane affects bacterial virulence and infection. Although a variety of Fe3+-transporters have been found in bacteria, their evolutionary processes are rarely studied. Pneumococcal iron ABC transporter (PitA), a highly conserved Fe3+-transporter in most pathogenic bacteria, influences the capsule formation and virulence of bacteria. However, multiple sequence alignment revealed that PitA is expressed in four different variants in bacteria, and the structural complexity of these variants increases progressively. To more efficiently import Fe3+ ions into bacterial cells, bacteria have evolved a fused PitA from two separately expressed PitA-1 (SPD_0227) and PitA-2 (SPD_0226) proteins. Further biochemical characterization indicated that both PitA-1 and PitA-2 have weaker Fe3+-binding ability than their protein complex. More importantly, Glutathione S-Transferase (GST) pull-down and isothermal titration calorimetry (ITC) detection showed that PitA-1 and PitA-2 interact with each other via Tyr111-Leu37, Asn112-Gln38, Asn103-Leu33, and Asn103-Thr34. Further molecular dynamics (MD) simulations demonstrated that this interaction in full-length PitA is stronger than that in the two individual proteins. Deletion of PitA family genes could lead to decrease in the ability of iron acquisition and of adhesion and invasion of S. pneumoniae. Our study revealed the evolving state and molecular mechanism of Fe3+-transporter PitAs in bacteria and provided important information for understanding the iron transportation mechanism in bacteria and designing new antibacterial drugs.
中文翻译:

PitAs在链球菌种铁运输中的进化及其分子机制
铁是几乎所有细菌必不可少的元素。位于细胞膜上的铁ATP结合盒(ABC)转运蛋白会影响细菌的毒力和感染。尽管在细菌中发现了各种Fe 3+转运蛋白,但很少研究它们的进化过程。肺炎球菌铁ABC转运蛋白(PitA)是大多数致病细菌中高度保守的Fe 3+转运蛋白,会影响细菌的荚膜形成和细菌的毒性。然而,多序列比对揭示了PitA在细菌的四个不同变体中表达,并且这些变体的结构复杂性逐渐增加。为了更有效地导入Fe 3+离子进入细菌细胞后,细菌从两种分别表达的PitA-1(SPD_0227)和PitA-2(SPD_0226)蛋白进化出融合的PitA。进一步的生化特征表明,PitA-1和PitA-2均比其蛋白质复合物具有更弱的Fe 3+结合能力。更重要的是,谷胱甘肽S-转移酶(GST)下拉和等温滴定热法(ITC)检测表明PitA-1和PitA-2通过Tyr111-Leu37,Asn112-Gln38,Asn103-Leu33和Asn103-Thr34。进一步的分子动力学(MD)模拟表明,全长PitA中的这种相互作用比两个单独的蛋白质中的相互作用更强。PitA家族基因的缺失可能导致铁的捕获能力下降,肺炎链球菌的粘附和侵袭能力下降。我们的研究揭示了Fe 3+转运PitAs在细菌中的演化状态和分子机理,为了解细菌中铁的转运机理和设计新的抗菌药物提供了重要的信息。











































 京公网安备 11010802027423号
京公网安备 11010802027423号