当前位置:
X-MOL 学术
›
Soft Matter
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of the formation of microphase separated water clusters in a water-mediated physical network of perfluoropolyether tetraol†
Soft Matter ( IF 2.9 ) Pub Date : 2018-02-09 00:00:00 , DOI: 10.1039/c7sm02181j Ashwini A. Deshpande 1, 2, 2, 3, 4 , Arun Torris A. T. 1, 2, 3 , Swagata Pahari 2, 3, 5 , Shamal K. Menon 1, 2, 3 , Manohar V. Badiger 1, 2, 2, 3, 4 , P. R. Rajamohanan 2, 2, 3, 4, 6 , Prakash P. Wadgaonkar 1, 2, 2, 3, 4 , Sudip Roy 2, 2, 3, 4, 5 , Claudio Tonelli 7, 8, 9
Soft Matter ( IF 2.9 ) Pub Date : 2018-02-09 00:00:00 , DOI: 10.1039/c7sm02181j Ashwini A. Deshpande 1, 2, 2, 3, 4 , Arun Torris A. T. 1, 2, 3 , Swagata Pahari 2, 3, 5 , Shamal K. Menon 1, 2, 3 , Manohar V. Badiger 1, 2, 2, 3, 4 , P. R. Rajamohanan 2, 2, 3, 4, 6 , Prakash P. Wadgaonkar 1, 2, 2, 3, 4 , Sudip Roy 2, 2, 3, 4, 5 , Claudio Tonelli 7, 8, 9
Affiliation
|
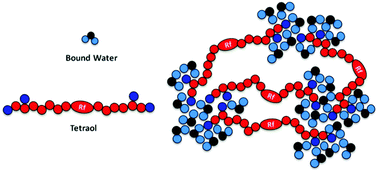
Perfluoropolyether tetraol (PFPE tetraol) possesses a hydrophobic perfluoropolyether chain in the backbone and two hydroxyl groups at each chain terminal, which facilitates the formation of hydrogen bonds with water molecules resulting in the formation an extended physical network. About 3 wt% water was required for the formation of the microphase separated physical network of PFPE tetraol. The mechanism responsible for the microphase separation of water clusters in the physical network was studied using a combination of techniques such as NMR spectroscopy, molecular dynamics (MD) simulations and DSC. MD simulation studies provided evidence for the formation of clusters in the PFPE tetraol physical network and the size of these clusters increased gradually with an increase in the extent of hydration. Both MD simulations and NMR spectroscopy studies revealed that these clusters position themselves away from the hydrophobic backbone or vice versa. The presence of intra- and inter-chain aggregation possibility among hydrophilic groups was evident. DSC results demonstrated the presence of tightly and loosely bound water molecules to the terminal hydroxyl groups of PFPE tetraol through hydrogen bonding. The data from all the three techniques established the formation of a physical network driven by hydrogen bonding between the hydrophilic end groups of PFPE tetraol and water molecules. The flexible nature of the PFPE tetraol backbone and its low solubility parameter favour clustering of water molecules at the terminal groups and result in the formation of a gel.
中文翻译:

在水介导的全氟聚醚四醇†物理网络中形成微相分离的水团簇的机制
全氟聚醚四醇(PFPE四醇)在主链上具有疏水性全氟聚醚链,并且在每个链末端具有两个羟基,这有助于与水分子形成氢键,从而形成扩展的物理网络。PFPE四元醇的微相分离物理网络的形成需要约3 wt%的水。使用诸如NMR光谱,分子动力学(MD)模拟和DSC之类的技术组合研究了物理网络中水簇微相分离的机理。MD模拟研究为PFPE四醇物理网络中簇的形成提供了证据,并且随着水合程度的增加,这些簇的大小逐渐增加。反之亦然。亲水基团之间存在链内和链间聚集的可能性是明显的。DSC结果证明通过氢键将水分子紧密和松散地结合到PFPE四醇的末端羟基上。来自所有三种技术的数据建立了由PFPE四元醇的亲水端基与水分子之间的氢键驱动的物理网络的形成。PFPE四醇主链的柔韧性和低溶解度参数有利于水分子在端基处聚集,并导致形成凝胶。
更新日期:2018-02-09
中文翻译:

在水介导的全氟聚醚四醇†物理网络中形成微相分离的水团簇的机制
全氟聚醚四醇(PFPE四醇)在主链上具有疏水性全氟聚醚链,并且在每个链末端具有两个羟基,这有助于与水分子形成氢键,从而形成扩展的物理网络。PFPE四元醇的微相分离物理网络的形成需要约3 wt%的水。使用诸如NMR光谱,分子动力学(MD)模拟和DSC之类的技术组合研究了物理网络中水簇微相分离的机理。MD模拟研究为PFPE四醇物理网络中簇的形成提供了证据,并且随着水合程度的增加,这些簇的大小逐渐增加。反之亦然。亲水基团之间存在链内和链间聚集的可能性是明显的。DSC结果证明通过氢键将水分子紧密和松散地结合到PFPE四醇的末端羟基上。来自所有三种技术的数据建立了由PFPE四元醇的亲水端基与水分子之间的氢键驱动的物理网络的形成。PFPE四醇主链的柔韧性和低溶解度参数有利于水分子在端基处聚集,并导致形成凝胶。











































 京公网安备 11010802027423号
京公网安备 11010802027423号