当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1-Dimensional Selective Nuclear Overhauser Effect NMR Spectroscopy To Characterize Products from a Two-Step Green Chemistry Synthesis
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2018-02-08 00:00:00 , DOI: 10.1021/acs.jchemed.7b00494 Russell Hopson 1 , Po Yin Bowie Lee 1 , Kathleen M. Hess 1
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2018-02-08 00:00:00 , DOI: 10.1021/acs.jchemed.7b00494 Russell Hopson 1 , Po Yin Bowie Lee 1 , Kathleen M. Hess 1
Affiliation
|
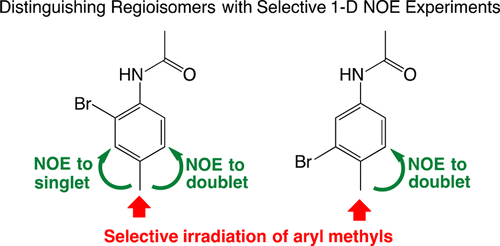
One dimensional (1-D) 1H and 13C NMR experiments are common tools used in undergraduate organic laboratories to characterize synthesized molecules. However, 1-D NMR spectra cannot always provide unambiguous structure determination. In research laboratories, advanced NMR spectroscopy methods are employed for structural assignments of synthesized molecules. This experiment is designed to introduce undergraduate students to the application of an advanced method of 1H NMR spectroscopy, the 1-D selective nuclear Overhauser effect (NOE) experiment, to fully characterize the structure of two compounds. The experiment features a two-step green chemistry synthesis to produce the two compounds. In the first step of the synthesis, 4-methylaniline (para-toluidine) is acetylated to form 4′-methylacetanilide (para-acetotoluidide). The 1-D selective NOE method is used to identify the two different methyl groups present in 4′-methylacetanilide and to assign the aromatic protons. For the second step of the synthesis, bromination of the aromatic ring of 4′-methylacetanilide is accomplished using Oxone and ammonium bromide in an aqueous solvent. The 1-D selective NOE method is applied to determine the regioselective product of the bromination reaction. The 1-D selective NOE experiment is readily accessible and easily implemented. Successful interpretation of the 1-D selective NOE results leaves students confident in making structural assignments that would otherwise be based solely on theory or software prediction.
中文翻译:

一维选择性核Overhauser效应NMR谱图,用于表征两步绿色化学合成的产物
一维(1-D)1 H和13 C NMR实验是大学有机实验室用来表征合成分子的常用工具。但是,一维NMR光谱不能总是提供明确的结构确定。在研究实验室中,先进的NMR光谱方法用于合成分子的结构分配。本实验旨在向大学生介绍一种先进的1 H NMR光谱方法(一维选择性核Overhauser效应(NOE)实验)的应用,以充分表征两种化合物的结构。该实验采用两步绿色化学合成法生产两种化合物。在合成的第一步中,4-甲基苯胺(对-甲苯胺)被乙酰化以形成4'-甲基乙酰苯胺(对乙酰丙酮化物)。一维选择性NOE方法用于鉴定4'-甲基乙酰苯胺中存在的两个不同的甲基,并指定芳香族质子。对于合成的第二步,在水性溶剂中使用Oxone和溴化铵完成4'-甲基乙酰苯胺的芳环的溴化。一维选择性NOE方法用于确定溴化反应的区域选择性产物。一维选择性NOE实验很容易获得和实施。一维选择性NOE结果的成功解释使学生有信心进行结构作业,而这些作业本来只能基于理论或软件预测。
更新日期:2018-02-08
中文翻译:

一维选择性核Overhauser效应NMR谱图,用于表征两步绿色化学合成的产物
一维(1-D)1 H和13 C NMR实验是大学有机实验室用来表征合成分子的常用工具。但是,一维NMR光谱不能总是提供明确的结构确定。在研究实验室中,先进的NMR光谱方法用于合成分子的结构分配。本实验旨在向大学生介绍一种先进的1 H NMR光谱方法(一维选择性核Overhauser效应(NOE)实验)的应用,以充分表征两种化合物的结构。该实验采用两步绿色化学合成法生产两种化合物。在合成的第一步中,4-甲基苯胺(对-甲苯胺)被乙酰化以形成4'-甲基乙酰苯胺(对乙酰丙酮化物)。一维选择性NOE方法用于鉴定4'-甲基乙酰苯胺中存在的两个不同的甲基,并指定芳香族质子。对于合成的第二步,在水性溶剂中使用Oxone和溴化铵完成4'-甲基乙酰苯胺的芳环的溴化。一维选择性NOE方法用于确定溴化反应的区域选择性产物。一维选择性NOE实验很容易获得和实施。一维选择性NOE结果的成功解释使学生有信心进行结构作业,而这些作业本来只能基于理论或软件预测。











































 京公网安备 11010802027423号
京公网安备 11010802027423号