Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
α-Actinin Anchors PSD-95 at Postsynaptic Sites.
Neuron ( IF 14.7 ) Pub Date : 2018-Mar-07 , DOI: 10.1016/j.neuron.2018.01.036 Lucas Matt 1 , Karam Kim 1 , Anne C Hergarden 1 , Tommaso Patriarchi 1 , Zulfiqar A Malik 2 , Deborah K Park 1 , Dhrubajyoti Chowdhury 1 , Olivia R Buonarati 1 , Peter B Henderson 1 , Çiğdem Gökçek Saraç 3 , Yonghong Zhang 4 , Durga Mohapatra 5 , Mary C Horne 2 , James B Ames 4 , Johannes W Hell 2
Neuron ( IF 14.7 ) Pub Date : 2018-Mar-07 , DOI: 10.1016/j.neuron.2018.01.036 Lucas Matt 1 , Karam Kim 1 , Anne C Hergarden 1 , Tommaso Patriarchi 1 , Zulfiqar A Malik 2 , Deborah K Park 1 , Dhrubajyoti Chowdhury 1 , Olivia R Buonarati 1 , Peter B Henderson 1 , Çiğdem Gökçek Saraç 3 , Yonghong Zhang 4 , Durga Mohapatra 5 , Mary C Horne 2 , James B Ames 4 , Johannes W Hell 2
Affiliation
|
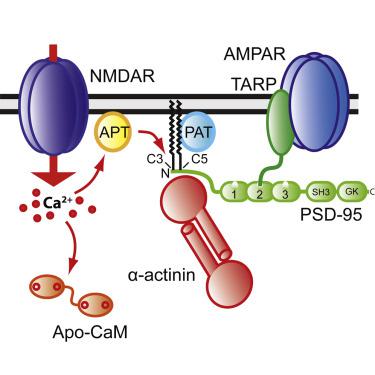
Despite the central role PSD-95 plays in anchoring postsynaptic AMPARs, how PSD-95 itself is tethered to postsynaptic sites is not well understood. Here we show that the F-actin binding protein α-actinin binds to the very N terminus of PSD-95. Knockdown (KD) of α-actinin phenocopies KD of PSD-95. Mutating lysine at position 10 or lysine at position 11 of PSD-95 to glutamate, or glutamate at position 53 or glutamate and aspartate at positions 213 and 217 of α-actinin, respectively, to lysine impairs, in parallel, PSD-95 binding to α-actinin and postsynaptic localization of PSD-95 and AMPARs. These experiments identify α-actinin as a critical PSD-95 anchor tethering the AMPAR-PSD-95 complex to postsynaptic sites.
中文翻译:

α-肌动蛋白将 PSD-95 锚定在突触后位点。
尽管 PSD-95 在锚定突触后 AMPAR 方面发挥着核心作用,但 PSD-95 本身如何与突触后位点结合尚不清楚。在这里,我们证明 F-肌动蛋白结合蛋白 α-肌动蛋白与 PSD-95 的 N 末端结合。 α-肌动蛋白表型的敲低 (KD) PSD-95 的 KD。将 PSD-95 位置 10 的赖氨酸或位置 11 的赖氨酸突变为谷氨酸,或突变为位置 53 的谷氨酸,或分别将 α-肌动蛋白位置 213 和 217 的谷氨酸和天冬氨酸突变为赖氨酸,同时损害 PSD-95 与α-辅肌动蛋白以及 PSD-95 和 AMPAR 的突触后定位。这些实验将 α-辅肌动蛋白确定为将 AMPAR-PSD-95 复合物束缚到突触后位点的关键 PSD-95 锚。
更新日期:2018-02-09
中文翻译:

α-肌动蛋白将 PSD-95 锚定在突触后位点。
尽管 PSD-95 在锚定突触后 AMPAR 方面发挥着核心作用,但 PSD-95 本身如何与突触后位点结合尚不清楚。在这里,我们证明 F-肌动蛋白结合蛋白 α-肌动蛋白与 PSD-95 的 N 末端结合。 α-肌动蛋白表型的敲低 (KD) PSD-95 的 KD。将 PSD-95 位置 10 的赖氨酸或位置 11 的赖氨酸突变为谷氨酸,或突变为位置 53 的谷氨酸,或分别将 α-肌动蛋白位置 213 和 217 的谷氨酸和天冬氨酸突变为赖氨酸,同时损害 PSD-95 与α-辅肌动蛋白以及 PSD-95 和 AMPAR 的突触后定位。这些实验将 α-辅肌动蛋白确定为将 AMPAR-PSD-95 复合物束缚到突触后位点的关键 PSD-95 锚。









































 京公网安备 11010802027423号
京公网安备 11010802027423号