当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Basis for Inhibitor-Induced Hydrogen Peroxide Production by Kynurenine 3-Monooxygenase
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2018-02-08 , DOI: 10.1016/j.chembiol.2018.01.008 Hyun Tae Kim , Byeong Kwan Na , Jiwoung Chung , Sulhee Kim , Sool Ki Kwon , Hyunju Cha , Jonghyeon Son , Joong Myung Cho , Kwang Yeon Hwang
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2018-02-08 , DOI: 10.1016/j.chembiol.2018.01.008 Hyun Tae Kim , Byeong Kwan Na , Jiwoung Chung , Sulhee Kim , Sool Ki Kwon , Hyunju Cha , Jonghyeon Son , Joong Myung Cho , Kwang Yeon Hwang
|
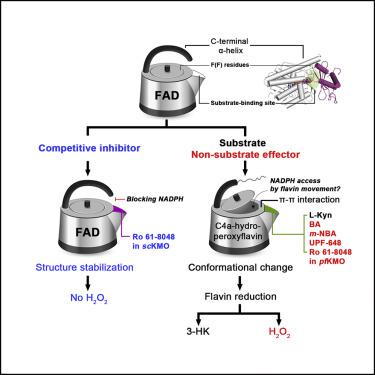
Kynurenine 3-monooxygenase (KMO) inhibitors have been developed for the treatment of neurodegenerative disorders. The mechanisms of flavin reduction and hydrogen peroxide production by KMO inhibitors are unknown. Herein, we report the structure of human KMO and crystal structures ofSaccharomyces cerevisiae(sc) andPseudomonas fluorescens(pf) KMO with Ro 61-8048. Proton transfer in the hydrogen bond network triggers flavin reduction inp-hydroxybenzoate hydroxylase, but the mechanism triggering flavin reduction in KMO is different. Conformational changes via π-π interactions between the loop above the flavin and substrate or non-substrate effectors lead to disorder of the C-terminal α helix inscKMO and shifts of domain III inpfKMO, stimulating flavin reduction. Interestingly, Ro 61-8048 has two different binding modes. It acts as a competitive inhibitor inscKMO and as a non-substrate effector inpfKMO. These findings provide understanding of the catalytic cycle of KMO and insight for structure-based drug design of KMO inhibitors.
中文翻译:

Kynurenine 3-单加氧酶抑制剂诱导的过氧化氢生产的结构基础
已开发了Kynurenine 3-单加氧酶(KMO)抑制剂,用于治疗神经退行性疾病。KMO抑制剂减少黄素和生成过氧化氢的机理尚不清楚。在此,我们报道了具有Ro 61-8048的人类KMO的结构以及酿酒酵母(sc)和荧光假单胞菌(pf)的KMO的晶体结构。氢键网络中的质子转移触发黄素还原对羟基苯甲酸羟化酶,但触发KMO中黄素还原的机理不同。通过黄素上方的环与底物或非底物效应子之间的π-π相互作用进行构象变化,会导致C末端α螺旋inscKMO紊乱和结构域III inpfKMO移位,从而刺激黄素减少。有趣的是,Ro 61-8048具有两种不同的绑定模式。它充当竞争性抑制剂inscKMO和非底物效应子inpfKMO。这些发现提供了对KMO催化循环的理解,并为KMO抑制剂的基于结构的药物设计提供了见识。
更新日期:2018-04-19
中文翻译:

Kynurenine 3-单加氧酶抑制剂诱导的过氧化氢生产的结构基础
已开发了Kynurenine 3-单加氧酶(KMO)抑制剂,用于治疗神经退行性疾病。KMO抑制剂减少黄素和生成过氧化氢的机理尚不清楚。在此,我们报道了具有Ro 61-8048的人类KMO的结构以及酿酒酵母(sc)和荧光假单胞菌(pf)的KMO的晶体结构。氢键网络中的质子转移触发黄素还原对羟基苯甲酸羟化酶,但触发KMO中黄素还原的机理不同。通过黄素上方的环与底物或非底物效应子之间的π-π相互作用进行构象变化,会导致C末端α螺旋inscKMO紊乱和结构域III inpfKMO移位,从而刺激黄素减少。有趣的是,Ro 61-8048具有两种不同的绑定模式。它充当竞争性抑制剂inscKMO和非底物效应子inpfKMO。这些发现提供了对KMO催化循环的理解,并为KMO抑制剂的基于结构的药物设计提供了见识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号