当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantum Dots Elicit Hepatotoxicity through Lysosome-Dependent Autophagy Activation and Reactive Oxygen Species Production
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2018-02-08 00:00:00 , DOI: 10.1021/acsbiomaterials.7b00824 Jiajun Fan 1, 2 , Shaofei Wang 1, 2 , Xuyao Zhang 1, 2 , Wei Chen 1, 2 , Yubin Li 1, 2 , Ping Yang 3 , Zhonglian Cao 3 , Yichen Wang 1, 2 , Weiyue Lu 2 , Dianwen Ju 1, 2
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2018-02-08 00:00:00 , DOI: 10.1021/acsbiomaterials.7b00824 Jiajun Fan 1, 2 , Shaofei Wang 1, 2 , Xuyao Zhang 1, 2 , Wei Chen 1, 2 , Yubin Li 1, 2 , Ping Yang 3 , Zhonglian Cao 3 , Yichen Wang 1, 2 , Weiyue Lu 2 , Dianwen Ju 1, 2
Affiliation
|
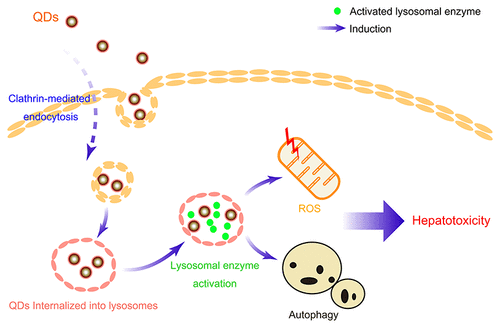
Quantum dots (QDs) were reported to be metabolized by the liver and demonstrated to be toxic in vitro and in vivo with unclear mechanisms, which largely limited their applications in the field of biomedical research. To improve their biosafety, the mechanism of how the QDs triggered hepatotoxicity was evaluated in this study. We found that CdTe/CdS QDs can trigger significant apoptosis-independent nanotoxicity after their uptake by liver cells and internalization into lysosomes. Besides, the lysosomal enzymes were abnormally activated after the QDs entered the lysosomes, which caused reactive oxygen species (ROS) production and autophagy activation. Importantly, inhibition of lysosomal enzymes not only rescued the viability of liver cells but also blocked the production of ROS and activation of autophagic flux, whereas inhibition of ROS and autophagy could ameliorate the hepatotoxicity induced by QDs but had no impact on the activity of lysosomal enzymes. Our results elucidate the relationship among the lysosomes, ROS, and autophagy in QDs-induced hepatotoxicity, which indicate that the QDs can elicit hepatotoxicity through lysosome-dependent autophagy activation and ROS production, highlighting an approach to improve the biosafety of QDs by lysosomal inhibition.
中文翻译:

量子点通过溶酶体依赖的自噬激活和活性氧的产生引起肝毒性
据报道,量子点(QD)被肝脏代谢,并且在体外和体内均表现出毒性,机制尚不清楚,这在很大程度上限制了其在生物医学研究领域的应用。为了提高其生物安全性,本研究评估了量子点如何触发肝毒性的机制。我们发现CdTe / CdS量子点在被肝细胞摄取并内化到溶酶体后,可以引发明显的细胞凋亡无关的纳米毒性。此外,量子点进入溶酶体后,溶酶体酶被异常激活,这导致了活性氧(ROS)的产生和自噬的激活。重要的是,抑制溶酶体酶不仅可以挽救肝细胞的活力,还可以阻止ROS的产生和自噬通量的激活,抑制ROS和自噬可以减轻QDs引起的肝毒性,但对溶酶体酶的活性没有影响。我们的结果阐明了QDs诱导的肝毒性中的溶酶体,ROS和自噬之间的关系,这表明QDs可以通过依赖于溶酶体的自噬激活和ROS产生而引起肝毒性,强调了通过溶酶体抑制来改善QDs的生物安全性的方法。
更新日期:2018-02-08
中文翻译:

量子点通过溶酶体依赖的自噬激活和活性氧的产生引起肝毒性
据报道,量子点(QD)被肝脏代谢,并且在体外和体内均表现出毒性,机制尚不清楚,这在很大程度上限制了其在生物医学研究领域的应用。为了提高其生物安全性,本研究评估了量子点如何触发肝毒性的机制。我们发现CdTe / CdS量子点在被肝细胞摄取并内化到溶酶体后,可以引发明显的细胞凋亡无关的纳米毒性。此外,量子点进入溶酶体后,溶酶体酶被异常激活,这导致了活性氧(ROS)的产生和自噬的激活。重要的是,抑制溶酶体酶不仅可以挽救肝细胞的活力,还可以阻止ROS的产生和自噬通量的激活,抑制ROS和自噬可以减轻QDs引起的肝毒性,但对溶酶体酶的活性没有影响。我们的结果阐明了QDs诱导的肝毒性中的溶酶体,ROS和自噬之间的关系,这表明QDs可以通过依赖于溶酶体的自噬激活和ROS产生而引起肝毒性,强调了通过溶酶体抑制来改善QDs的生物安全性的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号