当前位置:
X-MOL 学术
›
Nanoscale Horiz.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A programmable lipid-polymer hybrid nanoparticle system for localized, sustained antibiotic delivery to Gram-positive and Gram-negative bacterial biofilms
Nanoscale Horizons ( IF 8.0 ) Pub Date : 2018-02-08 00:00:00 , DOI: 10.1039/c7nh00167c Jong-Suep Baek 1, 2, 3, 4 , Chuan Hao Tan 1, 2, 3, 4, 5 , Noele Kai Jing Ng 2, 4, 5 , Yee Phan Yeo 2, 4, 5, 6, 7 , Scott A. Rice 2, 4, 5, 6, 7 , Say Chye Joachim Loo 1, 2, 3, 4, 5
Nanoscale Horizons ( IF 8.0 ) Pub Date : 2018-02-08 00:00:00 , DOI: 10.1039/c7nh00167c Jong-Suep Baek 1, 2, 3, 4 , Chuan Hao Tan 1, 2, 3, 4, 5 , Noele Kai Jing Ng 2, 4, 5 , Yee Phan Yeo 2, 4, 5, 6, 7 , Scott A. Rice 2, 4, 5, 6, 7 , Say Chye Joachim Loo 1, 2, 3, 4, 5
Affiliation
|
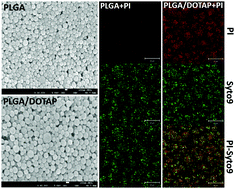
Bacteria enmeshed in an extracellular matrix, biofilms, exhibit enhanced antibiotic tolerance. Coupled with the rapid emergence of multidrug-resistant strains, the current cohorts of antibiotics are becoming ineffective. Alternative antimicrobial approaches are therefore urgently needed to overcome recalcitrant biofilm infections. Here, we propose the use of a non-toxic lipid-polymer hybrid nanoparticle (LPN) system composed of a solid polymer core (i.e. PLGA; poly lactic-co-glycolic acid) and a cationic lipid shell (i.e. DOTAP) for localized, sustained release of antimicrobial agents to bacterial biofilms. LPNs were synthesized through a simple, robust self-assembly approach. LPNs of uniform particle size (i.e. 100–130 nm), efficiently encapsulated (up to 95%) bioimaging molecules or antibiotics and provided controlled release of the latter. The cationic lipid coating enabled the LPN to anchor onto surfaces of a diverse range of Gram-positive and Gram-negative bacterial pathogens, either in the planktonic or biofilm form. Consistently, the LPN formulations reduced more than 95% of biofilm activity at concentrations that were 8 to 32-fold lower than free antibiotics. These data clearly indicate that these novel formulations could be a useful strategy to enhance the efficacy of antimicrobials against planktonic cells and biofilms of diverse species.
中文翻译:

可编程脂质-聚合物杂化纳米颗粒系统,用于将细菌持续,局部地递送至革兰氏阳性和革兰氏阴性细菌生物膜
细菌渗入细胞外基质,生物膜中,表现出增强的抗生素耐受性。加上多药耐药菌株的迅速出现,当前的抗生素组变得无效。因此,迫切需要替代的抗菌方法来克服顽强的生物膜感染。在这里,我们建议使用由固体聚合物核(即PLGA;聚乳酸-共-乙醇酸)和阳离子脂质壳(即DOTAP)组成的无毒脂质-聚合物杂化纳米颗粒(LPN)系统进行局部定位,抗菌剂向细菌生物膜的持续释放。LPN是通过简单,强大的自组装方法合成的。粒径均一的LPN(即100-130 nm),有效封装(高达95%)的生物成像分子或抗生素,并提供对后者的控制释放。阳离子脂质涂层使LPN可以锚定在浮游生物或生物膜形式的多种革兰氏阳性和革兰氏阴性细菌病原体的表面上。一致地,LPN制剂在浓度比游离抗生素低8到32倍的情况下降低了95%以上的生物膜活性。这些数据清楚地表明,这些新颖的制剂可能是增强抗微生物剂对多种物种的浮游细胞和生物膜的功效的有用策略。
更新日期:2018-02-08
中文翻译:

可编程脂质-聚合物杂化纳米颗粒系统,用于将细菌持续,局部地递送至革兰氏阳性和革兰氏阴性细菌生物膜
细菌渗入细胞外基质,生物膜中,表现出增强的抗生素耐受性。加上多药耐药菌株的迅速出现,当前的抗生素组变得无效。因此,迫切需要替代的抗菌方法来克服顽强的生物膜感染。在这里,我们建议使用由固体聚合物核(即PLGA;聚乳酸-共-乙醇酸)和阳离子脂质壳(即DOTAP)组成的无毒脂质-聚合物杂化纳米颗粒(LPN)系统进行局部定位,抗菌剂向细菌生物膜的持续释放。LPN是通过简单,强大的自组装方法合成的。粒径均一的LPN(即100-130 nm),有效封装(高达95%)的生物成像分子或抗生素,并提供对后者的控制释放。阳离子脂质涂层使LPN可以锚定在浮游生物或生物膜形式的多种革兰氏阳性和革兰氏阴性细菌病原体的表面上。一致地,LPN制剂在浓度比游离抗生素低8到32倍的情况下降低了95%以上的生物膜活性。这些数据清楚地表明,这些新颖的制剂可能是增强抗微生物剂对多种物种的浮游细胞和生物膜的功效的有用策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号