Molecular Catalysis ( IF 3.9 ) Pub Date : 2018-02-07 , DOI: 10.1016/j.mcat.2017.12.021 Juliana Tacacima , Silas Derenzo , Joao Guilherme Rocha Poco
|
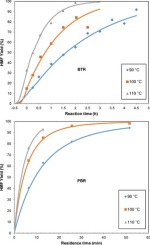
5-hydroxymethyl-2-furfural (HMF) is an important furanic compound that can be obtained from renewable raw materials. HMF has wide application in the industry, acting as an intermediate in the production of fine chemicals, polymer, biofuels, and pharmaceuticals. In this research, HMF was obtained from the catalytic fructose dehydration. Purolite® SGC650H, a gel-type strongly acidic resin, was applied as a catalyst using dimethylsulfoxide (DMSO) as a solvent. All experiments were carried out in a batch reactor (BTR) and a continuous packed bed reactor (PBR). The reactor performance data showed that increase in temperature resulted in higher HMF yield. In contrast, the higher the fructose concentration, the lower the HMF conversion. The HMF production rates in the PBR were higher because of the concentration of the catalyst. The rate constants were calculated by means of a pseudo-first-order reaction model, and the Arrhenius kinetic parameters obtained changed with regards to the reactants concentrations. The kinetic parameters displayed linear correlation in the Cremer-Constable diagram, which means that the system was under a kinetic compensation effect (KCE); therefore, the model was not suitable to represent the reaction. Langmuir-Hinshelwood (LHHW) models for rate equation were also tested. However all of them failed to adjust to the data, and thus a rate law was not determined. Due to the observed KCE, it was believed that there is at least a set of parallel dehydration reactions initiated from the different fructose isomers which has an influence on kinetic parameters.
中文翻译:

使用漂莱特从果糖HMF的合成® BTR和PBR反应器类型为动力学数据采集之间的比较:强酸催化剂
5-羟甲基-2-糠醛(HMF)是一种重要的呋喃化合物,可以从可再生原料中获得。HMF作为精细化学品,聚合物,生物燃料和药品生产的中间体,已在工业中得到广泛应用。在这项研究中,HMF是从果糖催化脱水中获得的。漂莱特®使用二甲基亚砜(DMSO)作为溶剂,将凝胶型强酸性树脂SGC650H用作催化剂。所有实验均在间歇反应器(BTR)和连续填充床反应器(PBR)中进行。反应器性能数据表明,温度升高导致更高的HMF收率。相反,果糖浓度越高,HMF转化率越低。由于催化剂的浓度,PBR中的HMF生产率较高。通过伪一级反应模型计算速率常数,并且获得的阿累尼乌斯动力学参数随反应物浓度而变化。动力学参数在Cremer-Constable图中显示出线性相关性,这意味着系统处于动力学补偿效应(KCE)下。所以,该模型不适合表示反应。还对Langmuir-Hinshelwood(LHHW)模型的速率方程进行了测试。但是,所有这些方法都无法适应数据,因此无法确定费率定律。由于观察到的KCE,据信存在至少一组由不同果糖异构体引发的平行脱水反应,这对动力学参数有影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号