当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring the ESIPT dynamical processes of two novel chromophores: symmetrical structure CHC and asymmetric structure CHN†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-02-08 00:00:00 , DOI: 10.1039/c7qo01127j Jiaojiao Hao 1, 2, 3, 4, 5 , Yang Yang 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-02-08 00:00:00 , DOI: 10.1039/c7qo01127j Jiaojiao Hao 1, 2, 3, 4, 5 , Yang Yang 1, 2, 3, 4, 5
Affiliation
|
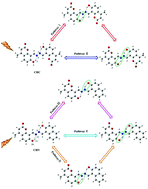
The detailed excited-state intramolecular proton transfer (ESIPT) dynamical processes of two novel chromophores, 8,8′-((1E,1E′)-hydrazine-1,2-diylidenebis(methanylylidene))bis-(7-hydroxy-4-methyl-2H-chromen-2-one) (CHC) (symmetrical structure) and 7-hydroxy-8-((E)-((E)-((2-hydroxynaphthalen-1-yl)-methylene)hydrazono)methyl)-4-methyl-2H-chromen-2-one (CHN) (asymmetric structure), which were synthesized in a previous study (Xiao et al., New. J. Chem., 2014, 38, 2386), were investigated by density functional theory (DFT) and time-dependent DFT (TDDFT) methods. Analysis of the bond lengths, angles and IR vibrational spectra confirmed that the intramolecular hydrogen bonds (HBs) of the CHC and CHN molecules were strengthened in the S1 state, which could facilitate ESIPT reactions. In addition, intramolecular charge transfer based on frontier molecular orbitals (MOs) and maps of the electron density difference between the S0 and S1 states indicated the possibility of ESIPT reactions for these two molecules. Moreover, to explore the detailed ESIPT dynamical processes of the CHC and CHN molecules, the potential energy surfaces (PESs) in the S0 and S1 states were constructed. The low excited-state potential barriers illustrated that both stepwise and simultaneous double ESIPT processes could occur for these two molecules. For the symmetrical structure CHC, two pathways of ESIPT processes existed as pathway I (stepwise double PT) and pathway II (simultaneous double PT). The relationship of the potential barriers was pathway II (4.71 kcal mol−1) < pathway I (6.15 and 7.62 kcal mol−1), which manifested that pathway II was more prone to double ESIPT for the CHC molecule. For the asymmetric structure CHN, three pathways of ESIPT processes existed as pathway III (stepwise double PT: firstly H8 from O7 to N9, and secondly H11 from O10 to N12), pathway IV (other stepwise double PT: firstly H11 from O10 to N12, and secondly H8 from O7 to N9) and pathway V (double PT). The relationship of the potential barriers was pathway III (2.67 and 6.75 kcal mol−1) < pathway IV (3.04 and 7.24 kcal mol−1) < pathway V (7.69 kcal mol−1), which indicated that pathway III was more susceptible to double ESIPT for the CHN molecule. Obviously, the ESIPT processes of the asymmetric structure CHN were more complicated than those of the symmetrical structure CHC.
中文翻译:

探索两种新型发色团的ESIPT动力学过程:对称结构CHC和不对称结构CHN †
两种新型生色团8,8'-(((1 E,1 E ')-肼-1,2-二亚甲基双(亚甲基亚烷基))双-(7-羟基)的详细激发态分子内质子转移(ESIPT)动力学过程-4-甲基-2 H-铬-2-(Cn)(对称结构)和7-羟基-8-((E)-((E)-((2-羟基萘-1-基)-亚甲基)(肼基)甲基)-4-甲基-2 H-铬-2--2-酮(CHN)(不对称结构),这是在先前的研究中合成的(Xiao等,New.J.Chem。,2014,38(2386),通过密度泛函理论(DFT)和时变DFT(TDDFT)方法进行了研究。对键长,键角和红外振动光谱的分析证实,CHC和CHN分子的分子内氢键(HBs)在S 1状态下得到增强,这可以促进ESIPT反应。此外,基于前沿分子轨道(MOs)的分子内电荷转移以及S 0和S 1状态之间的电子密度差图表明,这两种分子可能发生ESIPT反应。此外,为了探索CHC和CHN分子的详细ESIPT动力学过程,S 0和S 1中的势能面(PES)建立状态。低激发态势垒表明,这两个分子可能同时发生逐步的和同时的双重ESIPT过程。对于对称结构的CHC,存在ESIPT过程的两个路径,分别为路径I(逐步双PT)和路径II(同时双PT)。潜在障碍的关系为途径II(4.71 kcal mol -1)<途径I(6.15和7.62 kcal mol -1),这表明途径II对于CHC分子更倾向于加倍ESIPT。对于非对称结构CHN,存在ESIPT过程的三个途径,即途径III(逐步双PT:首先是H 8从O 7到N 9,其次是H 11从O10至N 12),路径IV(其他逐步双PT:首先是H 11从O 10到N 12,其次是H 8从O 7到N 9)和路径V(双PT)。势垒的关系为途径III(2.67和6.75 kcal mol -1)<途径IV(3.04和7.24 kcal mol -1)<途径V(7.69 kcal mol -1),这表明途径III更易受CHN分子的两倍ESIPT。显然,非对称结构CHN的ESIPT过程比对称结构CHC的ESIPT过程更为复杂。
更新日期:2018-02-08
中文翻译:

探索两种新型发色团的ESIPT动力学过程:对称结构CHC和不对称结构CHN †
两种新型生色团8,8'-(((1 E,1 E ')-肼-1,2-二亚甲基双(亚甲基亚烷基))双-(7-羟基)的详细激发态分子内质子转移(ESIPT)动力学过程-4-甲基-2 H-铬-2-(Cn)(对称结构)和7-羟基-8-((E)-((E)-((2-羟基萘-1-基)-亚甲基)(肼基)甲基)-4-甲基-2 H-铬-2--2-酮(CHN)(不对称结构),这是在先前的研究中合成的(Xiao等,New.J.Chem。,2014,38(2386),通过密度泛函理论(DFT)和时变DFT(TDDFT)方法进行了研究。对键长,键角和红外振动光谱的分析证实,CHC和CHN分子的分子内氢键(HBs)在S 1状态下得到增强,这可以促进ESIPT反应。此外,基于前沿分子轨道(MOs)的分子内电荷转移以及S 0和S 1状态之间的电子密度差图表明,这两种分子可能发生ESIPT反应。此外,为了探索CHC和CHN分子的详细ESIPT动力学过程,S 0和S 1中的势能面(PES)建立状态。低激发态势垒表明,这两个分子可能同时发生逐步的和同时的双重ESIPT过程。对于对称结构的CHC,存在ESIPT过程的两个路径,分别为路径I(逐步双PT)和路径II(同时双PT)。潜在障碍的关系为途径II(4.71 kcal mol -1)<途径I(6.15和7.62 kcal mol -1),这表明途径II对于CHC分子更倾向于加倍ESIPT。对于非对称结构CHN,存在ESIPT过程的三个途径,即途径III(逐步双PT:首先是H 8从O 7到N 9,其次是H 11从O10至N 12),路径IV(其他逐步双PT:首先是H 11从O 10到N 12,其次是H 8从O 7到N 9)和路径V(双PT)。势垒的关系为途径III(2.67和6.75 kcal mol -1)<途径IV(3.04和7.24 kcal mol -1)<途径V(7.69 kcal mol -1),这表明途径III更易受CHN分子的两倍ESIPT。显然,非对称结构CHN的ESIPT过程比对称结构CHC的ESIPT过程更为复杂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号