当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hypervalent Fluoroiodane‐Triggered Synthesis of Fluoro‐Azabenzoxazepines and Azaindoles
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2018-02-08 , DOI: 10.1002/ejoc.201800129 Christoph Brunner 1 , Anna Andries-Ulmer 1 , Gabriel M. Kiefl 1 , Tanja Gulder 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2018-02-08 , DOI: 10.1002/ejoc.201800129 Christoph Brunner 1 , Anna Andries-Ulmer 1 , Gabriel M. Kiefl 1 , Tanja Gulder 1
Affiliation
|
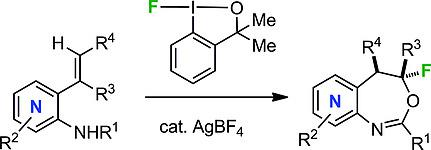
Fluorination reactions facilitated by hypervalent F‐iodanes have experienced vivid attention recently, since they often lead to novel, fluorinated scaffolds not accessible with common electrophilic fluorination reagents. The fluorocyclization of styrenes equipped with an amide functionality in the ortho position using F‐iodanes represents a transformation with unusual chemoselectivity. Within this context, the conversion of pyridine derivatives to fluoropyridyloxazepines, which constitutes a particular challenge due to the deactivating properties of the aza heterocycle, was accomplished in this work using the bench‐stable λ3‐F‐benzoiodoxole under Lewis acid catalysis. The versatility of the obtained F‐heterocycles as building blocks in organic synthesis was demonstrated by their straightforward conversion into azaindoles in a one‐pot, three‐step reaction sequence.
中文翻译:

氟氮杂氮杂苯并氮杂和氮杂吲哚的高价氟碘烷引发的合成
由高价F-碘酮促进的氟化反应最近引起了人们的广泛关注,因为它们经常导致新颖的氟化支架,而这是普通的亲电子氟化试剂无法达到的。在邻位使用F-碘酮对具有酰胺官能团的苯乙烯进行氟环化反应,可实现具有非同寻常的化学选择性的转化。在此范围内,吡啶衍生物对fluoropyridyloxazepines的转换,这构成了特别的挑战,由于氮杂杂环的钝化特性,在此工作中使用的实验用稳定λ完成3路易斯酸催化下的-F-苯并碘恶唑。所获得的F杂环作为有机合成的基本组成部分具有多功能性,可通过一锅三步反应序列将其直接转化为氮杂吲哚来证明。
更新日期:2018-05-15
中文翻译:

氟氮杂氮杂苯并氮杂和氮杂吲哚的高价氟碘烷引发的合成
由高价F-碘酮促进的氟化反应最近引起了人们的广泛关注,因为它们经常导致新颖的氟化支架,而这是普通的亲电子氟化试剂无法达到的。在邻位使用F-碘酮对具有酰胺官能团的苯乙烯进行氟环化反应,可实现具有非同寻常的化学选择性的转化。在此范围内,吡啶衍生物对fluoropyridyloxazepines的转换,这构成了特别的挑战,由于氮杂杂环的钝化特性,在此工作中使用的实验用稳定λ完成3路易斯酸催化下的-F-苯并碘恶唑。所获得的F杂环作为有机合成的基本组成部分具有多功能性,可通过一锅三步反应序列将其直接转化为氮杂吲哚来证明。











































 京公网安备 11010802027423号
京公网安备 11010802027423号