当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of the Cysteine 81 Residue of Macrophage Migration Inhibitory Factor as a Molecular Redox Switch
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-07 00:00:00 , DOI: 10.1021/acs.biochem.7b01156 Alexander Schinagl 1 , Randolf J. Kerschbaumer 1 , Nicolas Sabarth 1 , Patrice Douillard 1 , Peter Scholz 1 , Dirk Voelkel 1 , Julia C. Hollerweger 2 , Peter Goettig 2 , Hans Brandstetter 2 , Friedrich Scheiflinger 1 , Michael Thiele 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-07 00:00:00 , DOI: 10.1021/acs.biochem.7b01156 Alexander Schinagl 1 , Randolf J. Kerschbaumer 1 , Nicolas Sabarth 1 , Patrice Douillard 1 , Peter Scholz 1 , Dirk Voelkel 1 , Julia C. Hollerweger 2 , Peter Goettig 2 , Hans Brandstetter 2 , Friedrich Scheiflinger 1 , Michael Thiele 1
Affiliation
|
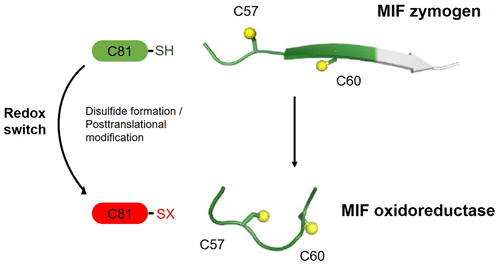
Macrophage migration inhibitory factor (MIF) is a pro-inflammatory and tumor-promoting cytokine that occurs in two redox-dependent immunologically distinct conformational isoforms. The disease-related structural isoform of MIF (oxMIF) can be specifically and predominantly detected in the circulation of patients with inflammatory diseases and in tumor tissue, whereas the ubiquitously expressed isoform of MIF (redMIF) is abundantly expressed in healthy and diseased subjects. In this article, we report that cysteine 81 within MIF serves as a “switch cysteine” for the conversion of redMIF to oxMIF. Modulating cysteine 81 by thiol reactive agents leads to significant structural rearrangements of the protein, resulting in a decreased β-sheet content and an increased random coil content, but maintaining the trimeric quaternary structure. This conformational change in the MIF molecule enables binding of oxMIF-specific antibodies BaxB01 and BaxM159, which showed beneficial activity in animal models of inflammation and cancer. Crystal structure analysis of the MIF-derived EPCALCS peptide, bound in its oxMIF-like conformation by the Fab fragment of BaxB01, revealed that this peptide adopts a curved conformation, making the central thiol protein oxidoreductase motif competent to undergo disulfide shuffling. We conclude that redMIF might reflect a latent zymogenic form of MIF, and formation of oxMIF leads to a physiologically relevant, i.e., enzymatically active, state.
中文翻译:

半胱氨酸81残留的巨噬细胞迁移抑制因子作为分子氧化还原开关的作用。
巨噬细胞迁移抑制因子(MIF)是一种促炎症和促肿瘤细胞因子,以两种氧化还原依赖性免疫学上独特的构象亚型发生。MIF(oxMIF)的与疾病相关的结构同工型可以在炎症性疾病患者的循环系统和肿瘤组织中特异性检测,而在健康和患病的受试者中普遍表达的MIF(redMIF)同工型丰富。在本文中,我们报道了MIF中的半胱氨酸81充当redMIF转换为oxMIF的“开关半胱氨酸”。通过硫醇反应剂调节半胱氨酸81会导致蛋白质的重大结构重排,从而导致β-片层含量降低和无规卷曲含量增加,但仍保持三聚体四级结构。MIF分子的这种构象变化使oxMIF特异性抗体BaxB01和BaxM159结合,在炎症和癌症的动物模型中显示出有益的活性。由BaxB01的Fab片段结合在其oxMIF样构象中的MIF衍生的EPCALCS肽的晶体结构分析表明,该肽采用弯曲构象,使中央硫醇蛋白氧化还原酶基序能够进行二硫键改组。我们得出的结论是,redMIF可能反映了MIF的潜在酶原形式,而oxMIF的形成则导致了生理上相关的状态,即具有酶促活性的状态。通过BaxB01的Fab片段结合在其oxMIF样构象中的MIF衍生的EPCALCS肽的晶体结构分析表明,该肽采用弯曲构象,使中心硫醇蛋白氧化还原酶基序能够进行二硫键改组。我们得出的结论是,redMIF可能反映了MIF的潜在酶原形式,而oxMIF的形成则导致了生理上相关的状态,即具有酶促活性的状态。通过BaxB01的Fab片段结合在其oxMIF样构象中的MIF衍生的EPCALCS肽的晶体结构分析表明,该肽采用弯曲构象,使中心硫醇蛋白氧化还原酶基序能够进行二硫键改组。我们得出的结论是,redMIF可能反映了MIF的潜在酶原形式,而oxMIF的形成则导致了生理上相关的状态,即具有酶促活性的状态。
更新日期:2018-02-07
中文翻译:

半胱氨酸81残留的巨噬细胞迁移抑制因子作为分子氧化还原开关的作用。
巨噬细胞迁移抑制因子(MIF)是一种促炎症和促肿瘤细胞因子,以两种氧化还原依赖性免疫学上独特的构象亚型发生。MIF(oxMIF)的与疾病相关的结构同工型可以在炎症性疾病患者的循环系统和肿瘤组织中特异性检测,而在健康和患病的受试者中普遍表达的MIF(redMIF)同工型丰富。在本文中,我们报道了MIF中的半胱氨酸81充当redMIF转换为oxMIF的“开关半胱氨酸”。通过硫醇反应剂调节半胱氨酸81会导致蛋白质的重大结构重排,从而导致β-片层含量降低和无规卷曲含量增加,但仍保持三聚体四级结构。MIF分子的这种构象变化使oxMIF特异性抗体BaxB01和BaxM159结合,在炎症和癌症的动物模型中显示出有益的活性。由BaxB01的Fab片段结合在其oxMIF样构象中的MIF衍生的EPCALCS肽的晶体结构分析表明,该肽采用弯曲构象,使中央硫醇蛋白氧化还原酶基序能够进行二硫键改组。我们得出的结论是,redMIF可能反映了MIF的潜在酶原形式,而oxMIF的形成则导致了生理上相关的状态,即具有酶促活性的状态。通过BaxB01的Fab片段结合在其oxMIF样构象中的MIF衍生的EPCALCS肽的晶体结构分析表明,该肽采用弯曲构象,使中心硫醇蛋白氧化还原酶基序能够进行二硫键改组。我们得出的结论是,redMIF可能反映了MIF的潜在酶原形式,而oxMIF的形成则导致了生理上相关的状态,即具有酶促活性的状态。通过BaxB01的Fab片段结合在其oxMIF样构象中的MIF衍生的EPCALCS肽的晶体结构分析表明,该肽采用弯曲构象,使中心硫醇蛋白氧化还原酶基序能够进行二硫键改组。我们得出的结论是,redMIF可能反映了MIF的潜在酶原形式,而oxMIF的形成则导致了生理上相关的状态,即具有酶促活性的状态。











































 京公网安备 11010802027423号
京公网安备 11010802027423号