当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activity of Topotecan toward the DNA/Topoisomerase I Complex: A Theoretical Rationalization
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-07 00:00:00 , DOI: 10.1021/acs.biochem.7b01297 Semiha Kevser Bali 1 , Antoine Marion 2 , Ilke Ugur 3 , Ayse Kumru Dikmenli 4 , Saron Catak 1 , Viktorya Aviyente 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-07 00:00:00 , DOI: 10.1021/acs.biochem.7b01297 Semiha Kevser Bali 1 , Antoine Marion 2 , Ilke Ugur 3 , Ayse Kumru Dikmenli 4 , Saron Catak 1 , Viktorya Aviyente 1
Affiliation
|
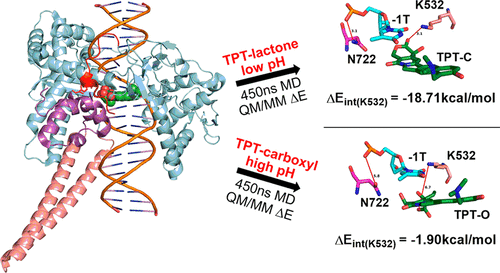
Topotecan (TPT) is a nontoxic anticancer drug characterized by a pH-dependent lactone/carboxyl equilibrium. TPT acts on the covalently bonded DNA/topoisomerase I (DNA/TopoI) complex by intercalating between two DNA bases at the active site. This turns TopoI into a DNA-damaging agent and inhibits supercoil relaxation. Although only the lactone form of the drug is active and effectively inhibits TopoI, both forms have been co-crystallized at the same location within the DNA/TopoI complex. To gain further insights into the pH-dependent activity of TPT, the differences between two TPT:DNA/TopoI complexes presenting either the lactone (acidic pH) or the carboxyl (basic pH) form of TPT were studied by means of molecular dynamic simulations, quantum mechanical/molecular mechanical calculations, and topological analysis. We identified two specific amino acids that have a direct relationship with the activity of the drug, i.e., lysine 532 (K532) and asparagine 722 (N722). K532 forms a stable hydrogen bond bridge between TPT and DNA only when the drug is in its active lactone form. The presence of the active drug triggers the formation of an additional stable interaction between DNA and protein residues, where N722 acts as a bridge between the two fragments, thus increasing the binding affinity of DNA for TopoI and further slowing the release of DNA. Overall, our results provide a clear understanding of the activity of the TPT-like class of molecules and can help in the future design of new anticancer drugs targeting topoisomerase enzymes.
中文翻译:

拓扑替康对DNA /拓扑异构酶I复合物的活性:理论上的合理化。
托泊替康(TPT)是一种无毒的抗癌药物,其特征在于pH依赖的内酯/羧基平衡。TPT通过在活性位点的两个DNA碱基之间插入来作用于共价键结合的DNA /拓扑异构酶I(DNA / TopoI)复合物。这将TopoI变成破坏DNA的试剂,并抑制了超螺旋松弛。尽管仅药物的内酯形式具有活性并有效抑制TopoI,但两种形式均已在DNA / TopoI复合物中的同一位置共结晶。为了进一步了解TPT的pH依赖性活性,通过分子动力学模拟研究了两种呈现TPT的内酯(酸性pH)或羧基(碱性pH)形式的TPT:DNA / TopoI复合物之间的差异,量子力学/分子力学计算和拓扑分析。我们鉴定了与药物活性直接相关的两个特定氨基酸,即赖氨酸532(K532)和天冬酰胺722(N722)。仅当药物呈活性内酯形式时,K532才能在TPT和DNA之间形成稳定的氢键桥。活性药物的存在触发了DNA与蛋白质残基之间另外稳定的相互作用的形成,其中N722充当两个片段之间的桥梁,因此增加了DNA对TopoI的结合亲和力并进一步减缓了DNA的释放。总体而言,我们的结果清楚地了解了类TPT类分子的活性,并有助于将来设计针对拓扑异构酶的新型抗癌药物。仅当药物呈活性内酯形式时,K532才能在TPT和DNA之间形成稳定的氢键桥。活性药物的存在触发了DNA与蛋白质残基之间另外稳定的相互作用的形成,其中N722充当两个片段之间的桥梁,因此增加了DNA对TopoI的结合亲和力并进一步减缓了DNA的释放。总体而言,我们的结果清楚地了解了类TPT类分子的活性,并有助于将来设计针对拓扑异构酶的新型抗癌药物。仅当药物呈活性内酯形式时,K532才能在TPT和DNA之间形成稳定的氢键桥。活性药物的存在触发了DNA与蛋白质残基之间另外稳定的相互作用的形成,其中N722充当两个片段之间的桥梁,因此增加了DNA对TopoI的结合亲和力并进一步减缓了DNA的释放。总体而言,我们的结果清楚地了解了类TPT类分子的活性,并有助于将来设计针对拓扑异构酶的新型抗癌药物。因此增加了DNA对TopoI的结合亲和力,并进一步减缓了DNA的释放。总体而言,我们的结果清楚地了解了类TPT类分子的活性,并有助于将来设计针对拓扑异构酶的新型抗癌药物。因此增加了DNA对TopoI的结合亲和力,并进一步减缓了DNA的释放。总体而言,我们的结果清楚地了解了类TPT类分子的活性,并有助于将来设计针对拓扑异构酶的新型抗癌药物。
更新日期:2018-02-07
中文翻译:

拓扑替康对DNA /拓扑异构酶I复合物的活性:理论上的合理化。
托泊替康(TPT)是一种无毒的抗癌药物,其特征在于pH依赖的内酯/羧基平衡。TPT通过在活性位点的两个DNA碱基之间插入来作用于共价键结合的DNA /拓扑异构酶I(DNA / TopoI)复合物。这将TopoI变成破坏DNA的试剂,并抑制了超螺旋松弛。尽管仅药物的内酯形式具有活性并有效抑制TopoI,但两种形式均已在DNA / TopoI复合物中的同一位置共结晶。为了进一步了解TPT的pH依赖性活性,通过分子动力学模拟研究了两种呈现TPT的内酯(酸性pH)或羧基(碱性pH)形式的TPT:DNA / TopoI复合物之间的差异,量子力学/分子力学计算和拓扑分析。我们鉴定了与药物活性直接相关的两个特定氨基酸,即赖氨酸532(K532)和天冬酰胺722(N722)。仅当药物呈活性内酯形式时,K532才能在TPT和DNA之间形成稳定的氢键桥。活性药物的存在触发了DNA与蛋白质残基之间另外稳定的相互作用的形成,其中N722充当两个片段之间的桥梁,因此增加了DNA对TopoI的结合亲和力并进一步减缓了DNA的释放。总体而言,我们的结果清楚地了解了类TPT类分子的活性,并有助于将来设计针对拓扑异构酶的新型抗癌药物。仅当药物呈活性内酯形式时,K532才能在TPT和DNA之间形成稳定的氢键桥。活性药物的存在触发了DNA与蛋白质残基之间另外稳定的相互作用的形成,其中N722充当两个片段之间的桥梁,因此增加了DNA对TopoI的结合亲和力并进一步减缓了DNA的释放。总体而言,我们的结果清楚地了解了类TPT类分子的活性,并有助于将来设计针对拓扑异构酶的新型抗癌药物。仅当药物呈活性内酯形式时,K532才能在TPT和DNA之间形成稳定的氢键桥。活性药物的存在触发了DNA与蛋白质残基之间另外稳定的相互作用的形成,其中N722充当两个片段之间的桥梁,因此增加了DNA对TopoI的结合亲和力并进一步减缓了DNA的释放。总体而言,我们的结果清楚地了解了类TPT类分子的活性,并有助于将来设计针对拓扑异构酶的新型抗癌药物。因此增加了DNA对TopoI的结合亲和力,并进一步减缓了DNA的释放。总体而言,我们的结果清楚地了解了类TPT类分子的活性,并有助于将来设计针对拓扑异构酶的新型抗癌药物。因此增加了DNA对TopoI的结合亲和力,并进一步减缓了DNA的释放。总体而言,我们的结果清楚地了解了类TPT类分子的活性,并有助于将来设计针对拓扑异构酶的新型抗癌药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号