Chemical Physics Letters ( IF 2.8 ) Pub Date : 2018-02-07 , DOI: 10.1016/j.cplett.2018.02.011 Vasilii I. Avdeev , Alexander F. Bedilo
|
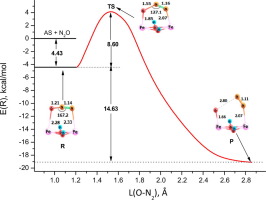
The electronic nature of sites over Fe-ferrierite zeolite stabilizing active α-oxygen is analyzed by the periodic DFT+U approach. It is shown that two antiferromagnetically coupled Fe2+ cations with bridging OH-bonds form a stable bi-nuclear site of the [Fe2+<2OH>Fe2+] doped FER complex. Frontier orbitals of this complex populated by two electrons with minority spins are localized in the bandgap. As a result, [Fe2+<2OH>Fe2+] unit acquires the properties of a binuclear Lewis acid dipolarophile for 1,3-dipole N2O. First reaction step of N2O decomposition follows the Huisgen‘s concept of the 1,3-dipolar cycloaddition concept followed by the formation of reactive oxygen species Fe-O.
中文翻译:

N 2 O分解在铁镁碱沸石的双核阳离子部位上形成活性氧:定期DFT + U研究
通过周期性DFT + U方法分析了铁镁碱沸石稳定活性α-氧上位点的电子性质。结果表明,两个具有桥接的OH键的反铁磁耦合的Fe 2+阳离子形成了[Fe 2+ 2OH 2 Fe 2+ ]掺杂的FER复合物的稳定的双核位点。由两个具有少数自旋的电子组成的复合物的前沿轨道位于带隙中。其结果是,的[Fe 2+ <2OH>的Fe 2+ ]单元获取的双核路易斯酸亲偶极的性质1,3-偶极Ñ 2 N个O.第一反应步骤2O分解遵循Huisgen的1,3-偶极环加成概念,然后形成活性氧Fe-O。











































 京公网安备 11010802027423号
京公网安备 11010802027423号