当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reduced 18F-Folate Conjugates as a New Class of PET Tracers for Folate Receptor Imaging
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-02-07 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00775 Silvan D. Boss 1 , Cristina Müller 2 , Klaudia Siwowska 2 , Josephine I. Büchel 1 , Raffaella M. Schmid 2 , Viola Groehn 3 , Roger Schibli 1, 2 , Simon M. Ametamey 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-02-07 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00775 Silvan D. Boss 1 , Cristina Müller 2 , Klaudia Siwowska 2 , Josephine I. Büchel 1 , Raffaella M. Schmid 2 , Viola Groehn 3 , Roger Schibli 1, 2 , Simon M. Ametamey 1
Affiliation
|
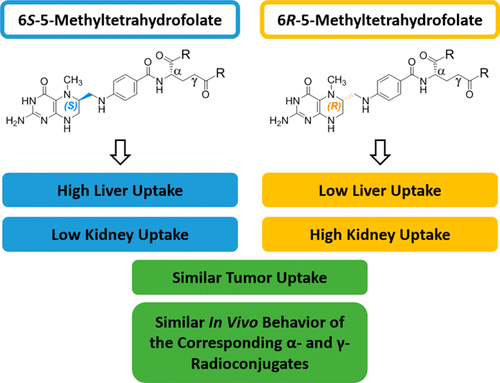
5-Methyltetrahydrofolate (5-MTHF), a reduced folate form, is the biologically active folate involved in many different metabolic processes. To date, there are no studies available in the literature on 18F-labeled 6S- and 6R-5-MTHF radiotracers for imaging folate receptor (FR)-α-positive tissues. Therefore, the goal of this study was to synthesize four 18F-labeled 5-MTHF derivatives conjugated at either the α- or γ-carboxylic functionality of glutamate and to assess their suitability for FR-targeting. Organic syntheses of the precursors and the four reference compounds, namely, 6S-α, 6S-γ, 6R-α, and 6R-γ-click-fluoroethyl-5-MTHF, were carried out in low to moderate overall chemical yields. The radiosyntheses of the α- and γ-conjugated 18F-labeled folate derivatives were accomplished in approximately 100 min, low radiochemical yields (1–7% d.c.) and high molar activities (139–245 GBq/μmol). Radiochemically pure tracers were obtained after the addition of a mixture of antioxidants consisting of sodium ascorbate and l-cysteine. In vitro, all four 5-MTHF conjugates showed similar binding affinities to FR-α (IC50 = 17.7–24.0 nM), whereas folic acid showed a significantly higher binding affinity to the FR-α. Cell uptake and internalization experiments with KB cells demonstrated specific uptake and internalization of the radiofolate conjugates. Metabolite studies in mice revealed high in vivo stability of the radiotracers in mice. Biodistribution and positron emission tomography (PET) imaging studies in FR-positive KB tumor-bearing mice demonstrated that the 6S- and 6R-5-MTHF conjugates exhibited a different accumulation pattern in various organs including the kidneys and the liver, whereas no significant differences in radioactivity accumulation in the kidneys and the liver were found for both the α- and γ-conjugated diastereoisomers. Despite the considerably lower binding affinities of the 5-MTHF derivatives compared to the corresponding folic acid conjugates similar high KB tumor uptake was observed for all the folate conjugates investigated (8–11% IA/g). Based on these results, we conclude that 18F-labeled 5-MTHF conjugates are a promising new class of radiotracers for targeting FR-positive tumor tissues.
中文翻译:

减少了18种F-叶酸酯共轭物,成为用于叶酸受体成像的新型PET示踪剂
5-甲基四氢叶酸(5-MTHF),一种还原的叶酸形式,是参与许多不同代谢过程的生物活性叶酸。迄今为止,在文献中还没有关于18 F标记的6 S-和6 R -5-MTHF放射性示踪剂用于叶酸受体(FR)-α阳性组织成像的研究。因此,本研究的目的是合成四种在谷氨酸的α-或γ-羧基官能团上缀合的18 F标记的5-MTHF衍生物,并评估其对FR靶向的适用性。前体和四种参考化合物(即6 S- α,6 S- γ,6 R- α和6 R)的有机合成-γ-单击-氟乙基-5-MTHF以低至中等的总化学产率进行。α-和γ-共轭的18 F标记的叶酸衍生物的放射性合成在大约100分钟内完成,放射化学产率低(直流1-7%),摩尔活性高(139-245 GBq /μmol)。加入由抗坏血酸钠和1-半胱氨酸组成的抗氧化剂混合物后,获得放射化学纯的示踪剂。在体外,所有四种5-MTHF共轭物均表现出与FR-α相似的结合亲和力(IC 50= 17.7–24.0 nM),而叶酸对FR-α的结合亲和力明显更高。KB细胞的细胞摄取和内在化实验证明了放射性叶酸偶联物的特异性摄取和内在化。在小鼠体内进行的代谢物研究表明,放射性示踪剂在小鼠体内具有很高的稳定性。FR阳性KB荷瘤小鼠的生物分布和正电子发射断层扫描(PET)成像研究表明6 S-和6 R-5-MTHF共轭物在包括肾脏和肝脏在内的各种器官中表现出不同的积累模式,而对于α-和γ-共轭非对映异构体,肾脏和肝脏中放射性积累没有显着差异。尽管与相应的叶酸结合物相比,5-MTHF衍生物的结合亲和力低得多,但对所有研究的叶酸结合物都观察到了相似的高KB肿瘤吸收(8-11%IA / g)。基于这些结果,我们得出结论,18 F标记的5-MTHF缀合物是针对FR阳性肿瘤组织的有希望的新型放射性示踪剂。
更新日期:2018-02-07
中文翻译:

减少了18种F-叶酸酯共轭物,成为用于叶酸受体成像的新型PET示踪剂
5-甲基四氢叶酸(5-MTHF),一种还原的叶酸形式,是参与许多不同代谢过程的生物活性叶酸。迄今为止,在文献中还没有关于18 F标记的6 S-和6 R -5-MTHF放射性示踪剂用于叶酸受体(FR)-α阳性组织成像的研究。因此,本研究的目的是合成四种在谷氨酸的α-或γ-羧基官能团上缀合的18 F标记的5-MTHF衍生物,并评估其对FR靶向的适用性。前体和四种参考化合物(即6 S- α,6 S- γ,6 R- α和6 R)的有机合成-γ-单击-氟乙基-5-MTHF以低至中等的总化学产率进行。α-和γ-共轭的18 F标记的叶酸衍生物的放射性合成在大约100分钟内完成,放射化学产率低(直流1-7%),摩尔活性高(139-245 GBq /μmol)。加入由抗坏血酸钠和1-半胱氨酸组成的抗氧化剂混合物后,获得放射化学纯的示踪剂。在体外,所有四种5-MTHF共轭物均表现出与FR-α相似的结合亲和力(IC 50= 17.7–24.0 nM),而叶酸对FR-α的结合亲和力明显更高。KB细胞的细胞摄取和内在化实验证明了放射性叶酸偶联物的特异性摄取和内在化。在小鼠体内进行的代谢物研究表明,放射性示踪剂在小鼠体内具有很高的稳定性。FR阳性KB荷瘤小鼠的生物分布和正电子发射断层扫描(PET)成像研究表明6 S-和6 R-5-MTHF共轭物在包括肾脏和肝脏在内的各种器官中表现出不同的积累模式,而对于α-和γ-共轭非对映异构体,肾脏和肝脏中放射性积累没有显着差异。尽管与相应的叶酸结合物相比,5-MTHF衍生物的结合亲和力低得多,但对所有研究的叶酸结合物都观察到了相似的高KB肿瘤吸收(8-11%IA / g)。基于这些结果,我们得出结论,18 F标记的5-MTHF缀合物是针对FR阳性肿瘤组织的有希望的新型放射性示踪剂。









































 京公网安备 11010802027423号
京公网安备 11010802027423号