当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Role of Planarity versus Nonplanarity in the Electronic Communication of TCAQ-Based Push-Pull Chromophores.
ChemPlusChem ( IF 3.0 ) Pub Date : 2018-03-02 , DOI: 10.1002/cplu.201700553 Raúl García 1 , Joaquín Calbo 2 , Rafael Viruela 2 , M Ángeles Herranz 1 , Enrique Ortí 2 , Nazario Martín 1, 3
ChemPlusChem ( IF 3.0 ) Pub Date : 2018-03-02 , DOI: 10.1002/cplu.201700553 Raúl García 1 , Joaquín Calbo 2 , Rafael Viruela 2 , M Ángeles Herranz 1 , Enrique Ortí 2 , Nazario Martín 1, 3
Affiliation
|
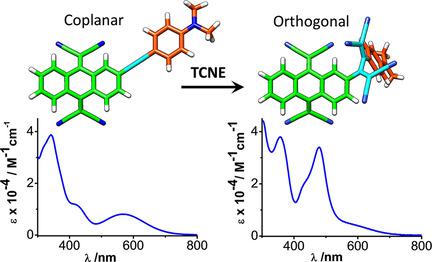
Donor-acceptor-substituted alkynes, endowed with 11,11,12,12-tetracyano-9,10-anthraquinodimethane (TCAQ) and N,N-dimethylaniline (DMA) units, have been further functionalized by a [2+2] cycloaddition with tetracyanoethylene (TCNE) followed by a subsequent retro-electrocyclization to form distorted nonplanar structures with bridging 1,1,4,4-tetracyanobuta-1,3-diene (TCBD) units. Comprehensive spectroscopic, electrochemical, and computational studies have been carried out to compare the electronic communication in planar (alkyne bridges) and nonplanar (TCBD bridges) TCAQ-based push-pull chromophores. Cyclic voltammetry and UV/Vis absorption measurements confirm the electronic communication between the TCAQ and DMA units despite the nonplanarity of the TCBD group. The experimental trends are strongly supported by density functional theory calculations, which further support the active electron-withdrawing role of the TCBD bridges. The novel push-pull TCAQ-based derivatives incorporating the TCBD bridge show a broad absorption in the whole visible range while the structures are highly distorted from planarity.
中文翻译:

平面性与非平面性在基于TCAQ的推挽发色团的电子通信中的作用。
具有11,11,12,12-四氰基-9,10-蒽醌二甲烷(TCAQ)和N,N-二甲基苯胺(DMA)单元的供体-受体取代的炔烃已通过[2 + 2]环加成反应进一步功能化先用四氰基乙烯(TCNE)进行随后的逆电环化,以形成具有桥接1,1,4,4-tetracyanobuta-1,3-diene(TCBD)单元的扭曲的非平面结构。已经进行了全面的光谱,电化学和计算研究,以比较基于平面(炔烃桥)和非平面(TCBD桥)TCAQ的推挽发色团的电子通信。循环伏安法和UV / Vis吸收测量证实了TCBD组的非平面性,但TCAQ和DMA单元之间存在电子通信。实验趋势得到密度泛函理论计算的有力支持,这进一步支持了TCBD桥的主动电子吸收作用。结合了TCBD桥的新型推挽基于TCAQ的衍生物在整个可见光范围内均显示出广泛的吸收性,同时该结构与平面度高度不相关。
更新日期:2018-03-02
中文翻译:

平面性与非平面性在基于TCAQ的推挽发色团的电子通信中的作用。
具有11,11,12,12-四氰基-9,10-蒽醌二甲烷(TCAQ)和N,N-二甲基苯胺(DMA)单元的供体-受体取代的炔烃已通过[2 + 2]环加成反应进一步功能化先用四氰基乙烯(TCNE)进行随后的逆电环化,以形成具有桥接1,1,4,4-tetracyanobuta-1,3-diene(TCBD)单元的扭曲的非平面结构。已经进行了全面的光谱,电化学和计算研究,以比较基于平面(炔烃桥)和非平面(TCBD桥)TCAQ的推挽发色团的电子通信。循环伏安法和UV / Vis吸收测量证实了TCBD组的非平面性,但TCAQ和DMA单元之间存在电子通信。实验趋势得到密度泛函理论计算的有力支持,这进一步支持了TCBD桥的主动电子吸收作用。结合了TCBD桥的新型推挽基于TCAQ的衍生物在整个可见光范围内均显示出广泛的吸收性,同时该结构与平面度高度不相关。









































 京公网安备 11010802027423号
京公网安备 11010802027423号