当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intra-arterial idarubicin_lipiodol without embolisation in hepatocellular carcinoma: The LIDA-B phase I trial
Journal of Hepatology ( IF 26.8 ) Pub Date : 2018-02-08 , DOI: 10.1016/j.jhep.2018.01.022 Boris Guiu , Jean-Louis Jouve , Antonin Schmitt , Anne Minello , Franck Bonnetain , Christophe Cassinotto , Lauranne Piron , Jean-Pierre Cercueil , Romaric Loffroy , Marianne Latournerie , Maëva Wendremaire , Côme Lepage , Mathieu Boulin
Journal of Hepatology ( IF 26.8 ) Pub Date : 2018-02-08 , DOI: 10.1016/j.jhep.2018.01.022 Boris Guiu , Jean-Louis Jouve , Antonin Schmitt , Anne Minello , Franck Bonnetain , Christophe Cassinotto , Lauranne Piron , Jean-Pierre Cercueil , Romaric Loffroy , Marianne Latournerie , Maëva Wendremaire , Côme Lepage , Mathieu Boulin
|
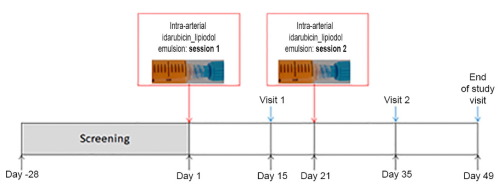
Idarubicin shows high cytotoxicity against hepatocellular carcinoma (HCC) cells, a high hepatic extraction ratio, and high lipophilicity leading to stable emulsions with lipiodol. A dose-escalation phase I trial of idarubicin_lipiodol (without embolisation) was conducted in patients with cirrhotic HCC to estimate the maximum-tolerated dose (MTD) and to assess the safety, efficacy, and pharmacokinetics of the drug, and the health-related quality of life achieved by patients. Patients underwent two sessions of treatment with a transarterial idarubicin_lipiodol emulsion without embolisation. The idarubicin dose was escalated according to a modified continuous reassessment method. The MTD was defined as the dose closest to that causing dose-limiting toxicity (DLT) in 20% of patients. A group of 15 patients were enrolled, including one patient at 10 mg, four patients at 15 mg, seven patients at 20 mg, and three patients at 25 mg. Only two patients experienced DLT: oedematous ascitic decompensation and abdominal pain at 20 and 25 mg, respectively. The calculated MTD of idarubicin was 20 mg. The most frequent grade ≥3 adverse events were biological. One month after the second session, the objective response rate was 29% (complete response, 0%; partial response, 29%) based on modified Response Evaluation Criteria In Solid Tumours. The median time to progression was 5.4 months [95% confidence limit (CI) 3.0–14.6 months] and median overall survival was 20.6 months (95% CI 5.7–28.7 months). Pharmacokinetic analysis of idarubicin showed that the mean C of idarubicin after intra-arterial injection of the idarubicin-lipiodol emulsion is approximately half the C after intravenous administration. Health-related quality of life results confirmed the good safety results associated with use of the drug. The MTD of idarubicin was 20 mg after two chemolipiodolisation sessions. Encouraging safety results, and patient responses and survival were observed. A phase II trial has been scheduled. There is a need for transarterial regimens that improve the responses and survival of patients with unresectable HCC. In this phase I trial, we showed that two sessions of treatment with a transarterial idarubicin_lipiodol emulsion without embolisation was well tolerated and gave promising efficacy in terms of tumour control and patient survival.
中文翻译:

肝细胞癌动脉内注射伊达比星碘油无栓塞治疗:LIDA-B I 期试验
Idarubicin 对肝细胞癌细胞 (HCC) 具有高细胞毒性、高肝提取率和高亲脂性,可与碘化油形成稳定的乳液。在肝硬化 HCC 患者中进行了伊达比星碘油(无栓塞)剂量递增 I 期试验,以估计最大耐受剂量 (MTD),并评估药物的安全性、有效性和药代动力学以及健康相关质量患者实现的生活。患者接受了两次经动脉伊达比星碘化油乳剂治疗,但未发生栓塞。根据改良的连续重新评估方法逐步增加伊达比星的剂量。 MTD 定义为最接近对 20% 患者造成剂量限制性毒性 (DLT) 的剂量。招募了 15 名患者,包括 1 名 10 mg 患者、4 名 15 mg 患者、7 名 20 mg 患者和 3 名 25 mg 患者。只有两名患者经历了 DLT:分别在 20 毫克和 25 毫克剂量时出现水肿性腹水失代偿和腹痛。计算得出的伊达比星 MTD 为 20 mg。最常见的 ≥3 级不良事件是生物性的。第二次治疗后一个月,根据修改后的实体瘤疗效评估标准,客观缓解率为 29%(完全缓解,0%;部分缓解,29%)。中位进展时间为 5.4 个月 [95% 置信限 (CI) 3.0–14.6 个月],中位总生存期为 20.6 个月(95% CI 5.7–28.7 个月)。伊达比星的药代动力学分析表明,动脉内注射伊达比星-碘化油乳剂后,伊达比星的平均Cmax约为静脉注射后Cmax的一半。与健康相关的生活质量结果证实了与使用该药物相关的良好安全性结果。两次碘化油治疗后,伊达比星的 MTD 为 20 mg。观察到了令人鼓舞的安全结果以及患者的反应和生存率。已安排进行第二阶段试验。需要改善不可切除的 HCC 患者的反应和生存的经动脉治疗方案。在这一 I 期试验中,我们表明,经动脉伊达比星碘化油乳剂进行两次治疗(无需栓塞)的耐受性良好,并且在肿瘤控制和患者生存方面具有良好的疗效。
更新日期:2018-02-08
中文翻译:

肝细胞癌动脉内注射伊达比星碘油无栓塞治疗:LIDA-B I 期试验
Idarubicin 对肝细胞癌细胞 (HCC) 具有高细胞毒性、高肝提取率和高亲脂性,可与碘化油形成稳定的乳液。在肝硬化 HCC 患者中进行了伊达比星碘油(无栓塞)剂量递增 I 期试验,以估计最大耐受剂量 (MTD),并评估药物的安全性、有效性和药代动力学以及健康相关质量患者实现的生活。患者接受了两次经动脉伊达比星碘化油乳剂治疗,但未发生栓塞。根据改良的连续重新评估方法逐步增加伊达比星的剂量。 MTD 定义为最接近对 20% 患者造成剂量限制性毒性 (DLT) 的剂量。招募了 15 名患者,包括 1 名 10 mg 患者、4 名 15 mg 患者、7 名 20 mg 患者和 3 名 25 mg 患者。只有两名患者经历了 DLT:分别在 20 毫克和 25 毫克剂量时出现水肿性腹水失代偿和腹痛。计算得出的伊达比星 MTD 为 20 mg。最常见的 ≥3 级不良事件是生物性的。第二次治疗后一个月,根据修改后的实体瘤疗效评估标准,客观缓解率为 29%(完全缓解,0%;部分缓解,29%)。中位进展时间为 5.4 个月 [95% 置信限 (CI) 3.0–14.6 个月],中位总生存期为 20.6 个月(95% CI 5.7–28.7 个月)。伊达比星的药代动力学分析表明,动脉内注射伊达比星-碘化油乳剂后,伊达比星的平均Cmax约为静脉注射后Cmax的一半。与健康相关的生活质量结果证实了与使用该药物相关的良好安全性结果。两次碘化油治疗后,伊达比星的 MTD 为 20 mg。观察到了令人鼓舞的安全结果以及患者的反应和生存率。已安排进行第二阶段试验。需要改善不可切除的 HCC 患者的反应和生存的经动脉治疗方案。在这一 I 期试验中,我们表明,经动脉伊达比星碘化油乳剂进行两次治疗(无需栓塞)的耐受性良好,并且在肿瘤控制和患者生存方面具有良好的疗效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号