当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Remarkable Influence of the Trifluoromethyl Group on the Reactions of -Mercaptoalcohols with Fluorinated -Bromoenones
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2018-04-06 , DOI: 10.1002/ejoc.201701752 Emilia Obijalska 1 , Maria Pawelec 1 , Grzegorz Mlostoń 1 , Antonella Capperucci 2 , Damiano Tanini 2 , Heinz Heimgartner 3
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2018-04-06 , DOI: 10.1002/ejoc.201701752 Emilia Obijalska 1 , Maria Pawelec 1 , Grzegorz Mlostoń 1 , Antonella Capperucci 2 , Damiano Tanini 2 , Heinz Heimgartner 3
Affiliation
|
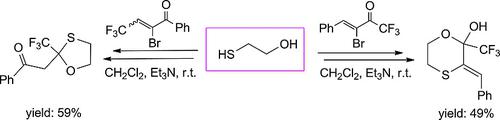
Isomeric fluorinated α‐bromoenones react with dinucleophilic β‐mercaptoalcohols in CH2Cl2 at room temperature in the presence of Et3N in a multistep process. Depending on the position of the CF3 group, different O,S‐heterocycles or non‐cyclic products were obtained. With 3‐bromo‐1,1,1‐trifluorobut‐3‐en‐2‐ones derivatives of 1,4‐oxathianes were formed, but isomeric 2‐bromo‐4,4,4‐trifluorobut‐2‐en‐1‐ones yielded 1,3‐oxathiolanes or non‐cyclic sulfides. The thia‐Michael addition is proposed as the initial step of the reaction, and the final heterocyclization is governed by the location of the CF3 group.
中文翻译:

三氟甲基对-巯基醇与氟化α-溴烯酮反应的显着影响
在室温下,在 Et3N 存在下,异构氟化 α-溴烯酮在 CH2Cl2 中与双亲核 β-巯基醇在多步过程中反应。根据 CF3 基团的位置,得到不同的 O,S-杂环或非环状产物。与 3-bromo-1,1,1-trifluorobut-3-en-2-ones 形成 1,4-oxathianes 的衍生物,但异构体 2-bromo-4,4,4-trifluorobut-2-en-1-生成 1,3-氧杂硫杂环戊烷或非环状硫化物。硫杂-迈克尔加成被提议作为反应的初始步骤,最终的杂环化由 CF3 基团的位置控制。
更新日期:2018-04-06
中文翻译:

三氟甲基对-巯基醇与氟化α-溴烯酮反应的显着影响
在室温下,在 Et3N 存在下,异构氟化 α-溴烯酮在 CH2Cl2 中与双亲核 β-巯基醇在多步过程中反应。根据 CF3 基团的位置,得到不同的 O,S-杂环或非环状产物。与 3-bromo-1,1,1-trifluorobut-3-en-2-ones 形成 1,4-oxathianes 的衍生物,但异构体 2-bromo-4,4,4-trifluorobut-2-en-1-生成 1,3-氧杂硫杂环戊烷或非环状硫化物。硫杂-迈克尔加成被提议作为反应的初始步骤,最终的杂环化由 CF3 基团的位置控制。









































 京公网安备 11010802027423号
京公网安备 11010802027423号